Preparation method of immune cell exosome carrying chimeric antigen receptor and application thereof
A technology of immune cells and exosomes, applied in the field of biomedicine, can solve the problems of non-specific targeting, dangerous clinical treatment, and no tumor lethality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1. Preparation of CAR exosomes derived from CAR-T cells
[0066]1) Whole gene synthesis including the CAR sequence of the anti-EGFR single-chain antibody (commissioned by Suzhou Jinweizhi Biotechnology Co., Ltd.), where the single-chain antibody sequence is derived from the anti-EGFR antibody cetuximab (Li et al., 2005, Structural basis for inhibition of the epidermal growth factor receptor by cetuximab, Cancer Cell, 7:301-311), the specific structure of CAR includes: anti-EGFR single-chain antibody scFv-CD8α hinge region and transmembrane region-4-1BB co-activation domain and CD3ζ signaling molecule cytoplasm inner segment. The specific sequence is roughly consistent with that reported in the literature Johnson L A, etal. Science translational medicine, 2015, 7 (275) (except scFv). In order to facilitate detection, a Myc tag was inserted between the scFv and the hinge region. The position of the tag was the same as that in the literature Chu J, et al.CS1-speci...
Embodiment 2
[0078] Example 2: CAR exosomes inhibit the viability of EGFR-positive MDA-MB-231 and HCC827 cells
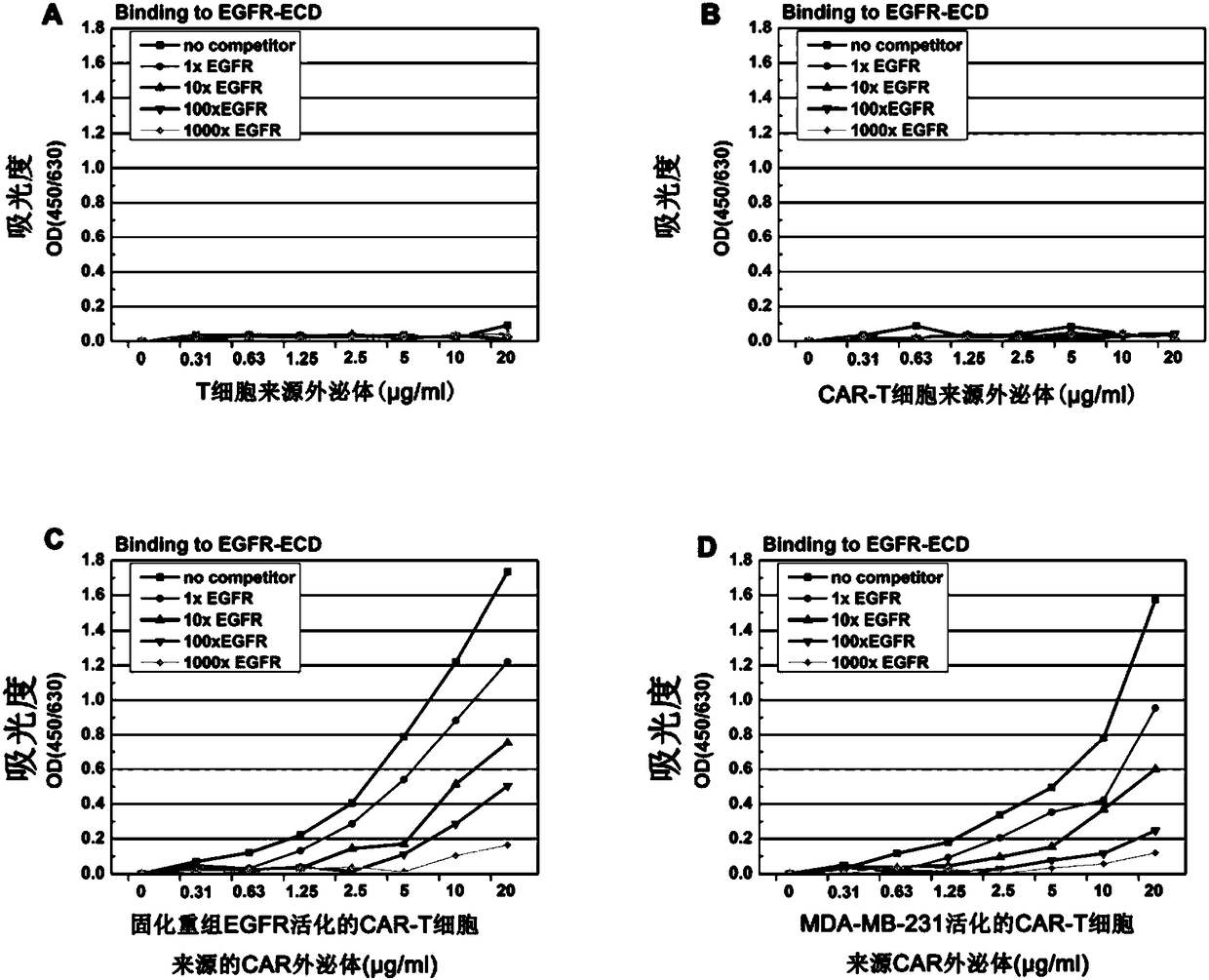
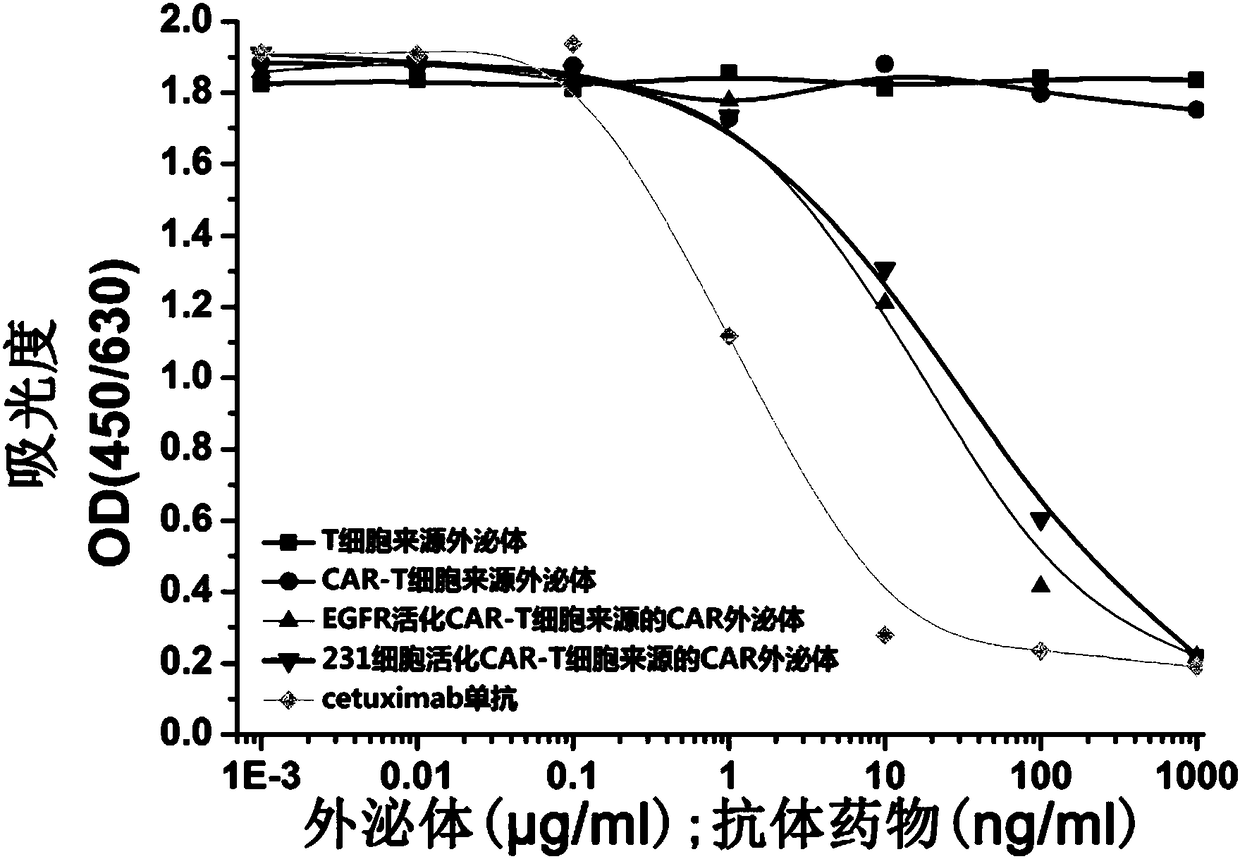
[0079] Take MDA-MB-231 and HCC827 cells (ATCC) in good growth state, and adjust the cell concentration to 5×10 3 / ml, seeded in 96-well cell culture plate, 200μl / well, at 37°C, 5% CO 2 After culturing in the incubator for 24 hours, EGF with a final concentration of 5 nmol and exosomes with different concentration gradients were added to the culture medium, and cetuximab antibody drug was used as a control (Cetuximab was purchased from Merck). After 4 days, the cell viability was measured by CellTiter -Glo Luminescent Cell Viability Assay kit (Promega, Madison, WI) detection. Experimental results such as image 3 shown. The experimental results showed that CAR exosomes could significantly inhibit the viability of MDA-MB-231 and HCC827 cells (P Figure 4 ).
Embodiment 3
[0080] Example 3: Experiment of CAR exosomes inhibiting tumor growth in vivo
[0081] In order to detect the anti-tumor activity of CAR exosomes in vivo, HCC827 and MDA-231 cells were firstly inoculated subcutaneously on the right flank of BALB / c nude mice (Experimental Animal Center, Chinese Academy of Sciences), and 3500 mg / kg of CAR was injected into the tail vein after tumor formation. Exosomes and antibody drug cetuximab (10 mg / kg) were injected once a week, and the mice were sacrificed until the tumor was too large. The length and width of the tumor were measured every day, and the tumor volume was calculated.
[0082] tumor growth curve Figure 5 shown. The results showed that the tumor growth rate of the activated CAR exosome treatment group was significantly smaller than that of the cetuximab treatment group (after 40 days, P<0.01, Bonferroni test).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com