CD22 protein targeting antibody, chimeric antigen receptor and drug
A chimeric antigen receptor and protein technology, which is applied in the fields of antibodies targeting CD22 protein, chimeric antigen receptors and drugs, can solve the problems of few types of CD22 antibodies, unsatisfactory tumor cell specific binding ability and lethality, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1. Phage library panning
[0040] Biotinylated hCD22 protein was used as the panning antigen for the fully human antibody library. First, block the phage antibody with blocking solution (PBST / 5% skimmed milk powder) at room temperature for 2 hours. The amount of phage input is 1012 pfu, then add 10 μg antigen, incubate at room temperature for 1 hour, and add 50 μl pre-blocked bacteriophage after incubation. M-280Streptavidin magnetic beads, incubated at room temperature for 30min.
[0041] First wash off the unbound phages with PBST, then elute the phages bound to the magnetic beads with 0.1M HCl-Glycine, then neutralize the eluate with Tris-HCl, and take part of the phages to infect the large intestine in the logarithmic growth phase Bacillus TG1, the collected phages were used for the next round of panning.
[0042] Gradually increase the screening intensity of each round, and stop panning when the enrichment degree reaches more than 100 times.
[0043] 2. Use phage...
Embodiment 2
[0059] Detect the binding force of the CD22 antibody obtained in Example 1 and hCD22 protein
[0060] Detection method:
[0061] (1) For hCD22 protein coating, dilute from 1ìg / ml, 3-fold serial dilution, a total of 7 gradients, coat Costar-9018 microtiter plate with 100ìl hCD22 protein dilution, overnight at 4°C.
[0062] (2) After blocking with 3% BSA at room temperature for 2 hours, add the phage supernatant (1010 pfu) corresponding to CD22 antibody, and incubate at room temperature for 2 hours.
[0063] (3) After washing away the unbound phage, add Ml3 Bacteriophage antibody (HRP), and incubate at 4°C for 1 hour. After washing, TMB chromogenic solution was added to develop color, and 2M HCl was used to terminate the reaction.
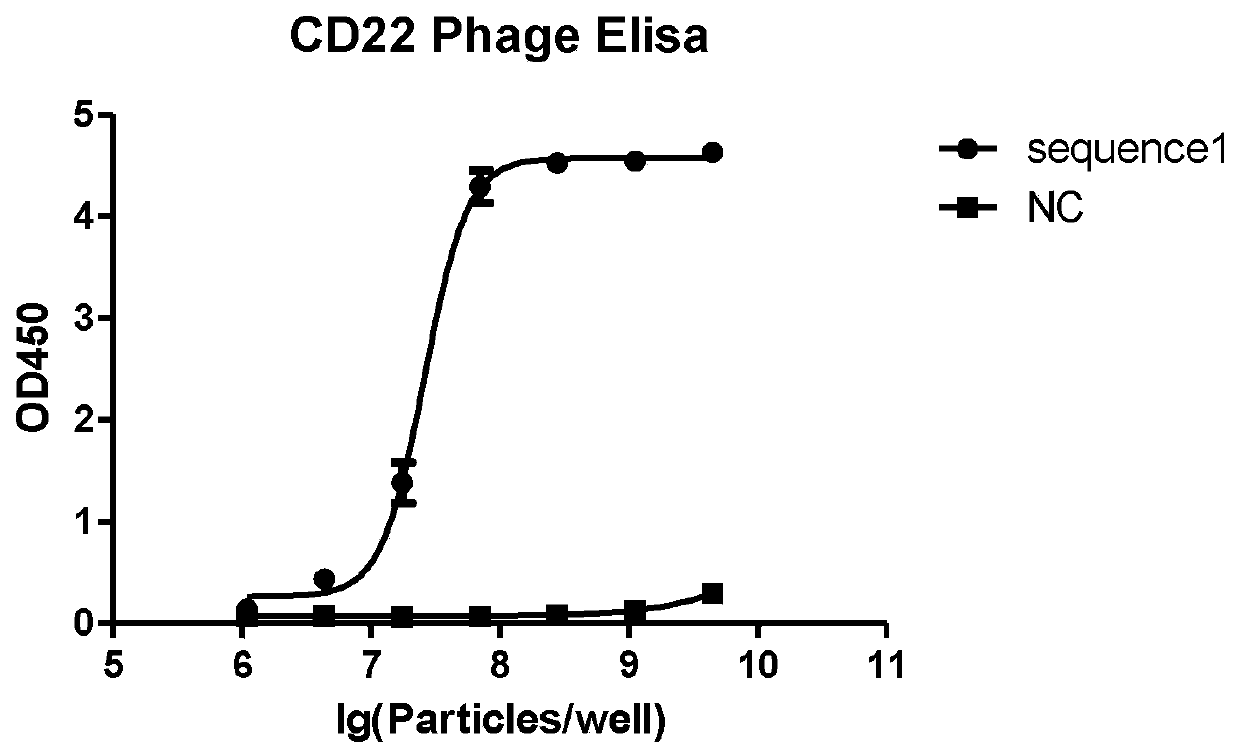
[0064] (4) Read at 450nm with a microplate reader, see the results figure 1 .
[0065] from figure 1 From the results, it can be seen that the phage expressing the CD22 antibody has a good binding ability to the hCD22 protein, showing an S curve...
Embodiment 3
[0067] Construction of chimeric antigen receptor expression vector
[0068] Build method:
[0069] (1) Whole gene synthesis: signal peptide, CD22 antibody light chain variable region, linker, CD22 antibody heavy chain variable region, hinge region (hinge), CD8α transmembrane domain (TM), 4-1BB co-stimulatory signal transduction region and the CD3ζ signaling domain. The above sequences were sequentially connected to obtain the CD22 chimeric antigen receptor expression cassette, which was named: CD22 chimeric antigen receptor expression cassette, and the Kozac sequence was introduced at the front end of the expression cassette. The expression cassette structure is as follows: figure 2 shown.
[0070] The sequence of each element of the CD22 chimeric antigen receptor expression cassette is as follows:
[0071] The amino acid sequence of the signal peptide (Leader) is shown in SEQ ID NO.1, specifically as follows:
[0072] MLLLVTSLLLCELPHPAFLLIP.
[0073] Its corresponding n...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com