Polypeptide helical conformation stabilizing method
A technology of helical conformation and linear peptides, which is applied in peptide preparation methods, chemical instruments and methods, peptides, etc., can solve problems such as unfavorable optimization of polypeptide structure and function, unfavorable optimization of peptide activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
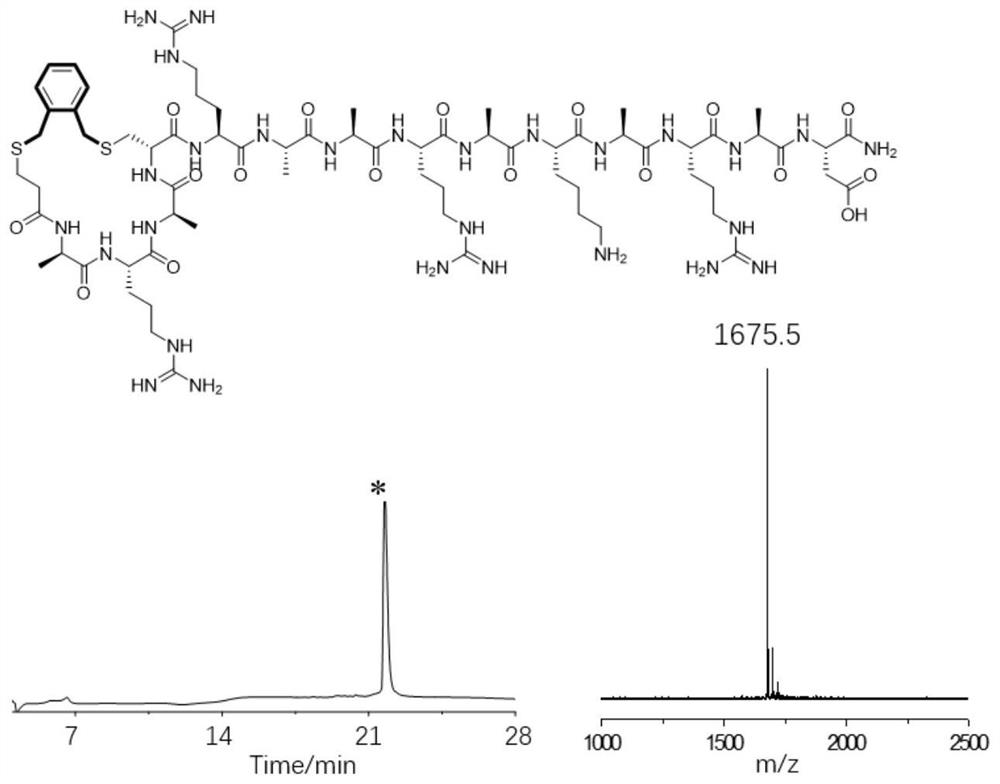
[0034] Example 1: Preparation of a cross-linked helix-stabilized polypeptide between the N-terminus of the polypeptide and the sulfhydryl group at the 4th side chain of the N-terminus
[0035]The fourth amino acid Ala at the N-terminal of the model peptide was mutated to Cys, and a new sequence peptide was designed, which was ARACRAARAKARAD. Through mature Fmoc solid-phase synthesis, we can obtain this linear peptide. The specific synthesis process of solid-phase synthesis of linear peptides includes the following steps:
[0036] (1) Swelling Rink Amide-AM Resin (loading capacity 0.33mmol / g, 30μmol): 90mg of resin was added to the solid phase synthesis tube, 2mL of DMF solution was added, and the reaction was shaken at room temperature for 15min;
[0037] (2) Fmoc deprotection: Add 1 mL of 20% piperidine / DMF mixed solution to the synthesis tube, react at room temperature for 10 min, discard the solution, then add 1 mL of 20% piperidine / DMF mixed solution to the synthesis tube...
Embodiment 2
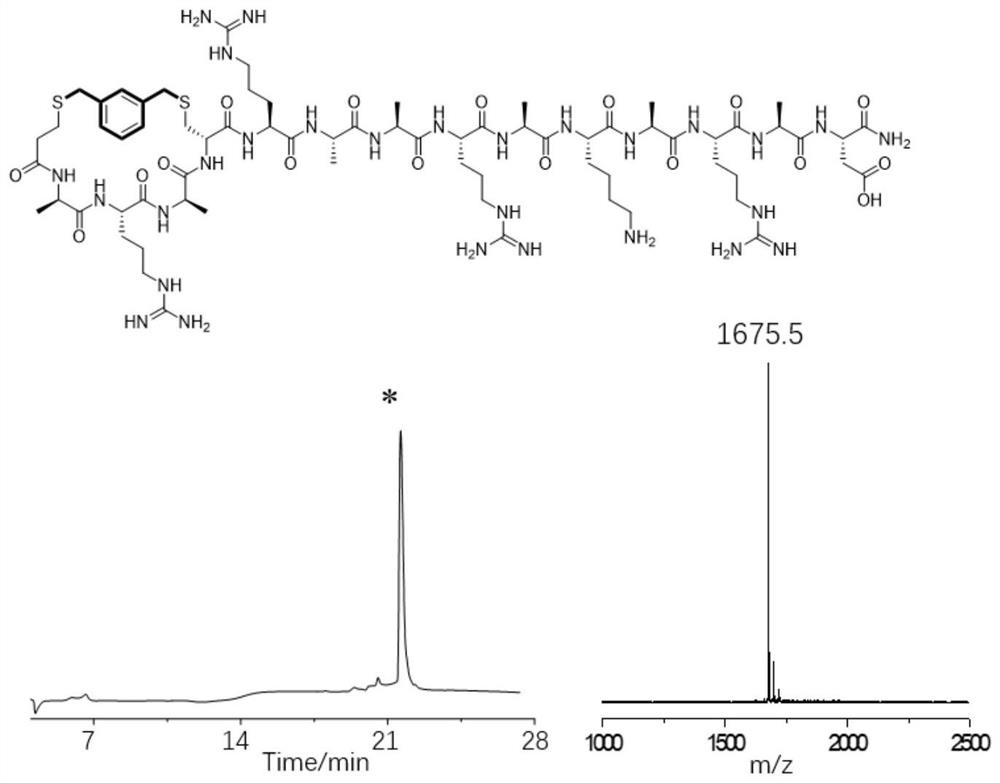
[0044] Example 2: Preparation of a cross-linked helix-stabilized polypeptide between the N-terminus of the polypeptide and the 5th side chain sulfhydryl group at the N-terminus
[0045] The fifth amino acid Arg at the N-terminal of the model peptide was mutated to Cys, and a new sequence peptide was designed, which was ARAACAARAKARAD. Through mature Fmoc solid-phase synthesis, we can obtain this linear peptide. The specific synthesis process of solid-phase synthesis of linear peptides includes the following steps:
[0046] (1) Swelling Rink Amide-AM Resin (loading capacity 0.33mmol / g, 30μmol): 90mg of resin was added to the solid phase synthesis tube, 2mL of DMF solution was added, and the reaction was shaken at room temperature for 15min;
[0047] (2) Fmoc deprotection: Add 1 mL of 20% piperidine / DMF mixed solution to the synthesis tube, react at room temperature for 10 min, discard the solution, then add 1 mL of 20% piperidine / DMF mixed solution to the synthesis tube for 10...
Embodiment 3
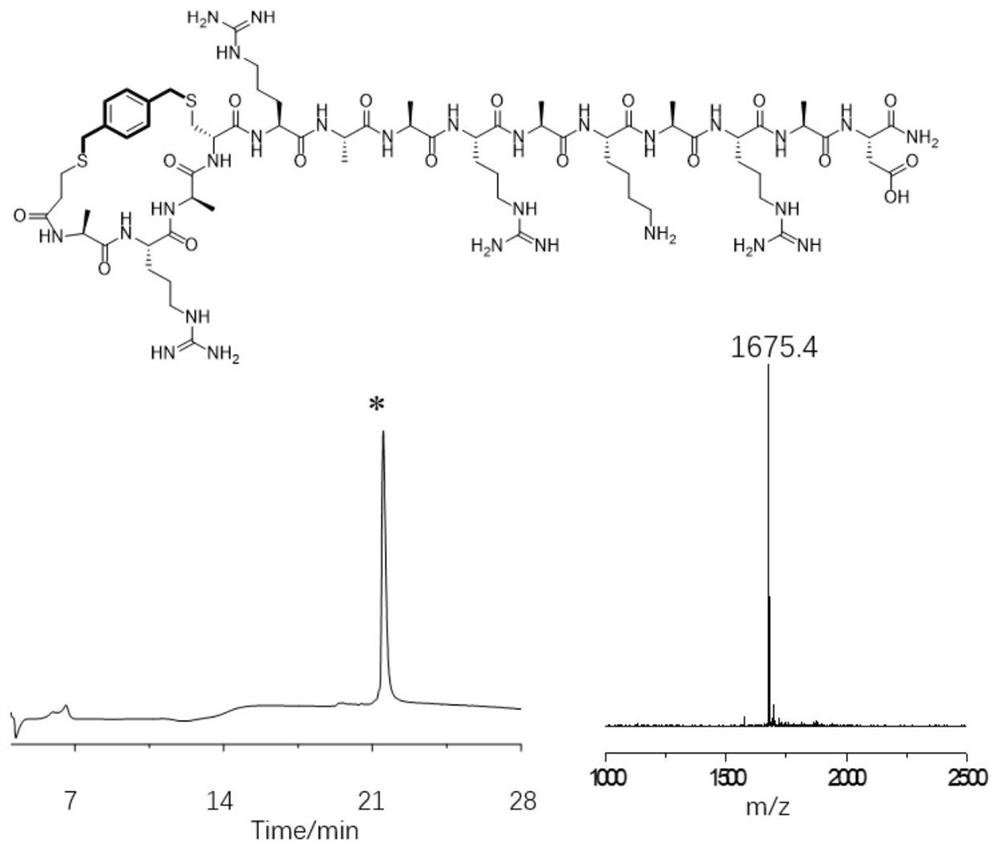
[0054] Example 3: Preparation of a cross-linked helix-stabilized polypeptide between the N-terminus of the polypeptide and the 8th side chain sulfhydryl group at the N-terminus
[0055] The 8th amino acid Arg at the N-terminal of the model peptide was mutated to Cys, and a new sequence peptide was designed, which was ARAARAACAKARAD. Through mature Fmoc solid-phase synthesis, we can obtain this linear peptide. The specific synthesis process of solid-phase synthesis of linear peptides includes the following steps:
[0056] (1) Swelling Rink Amide-AM Resin (loading capacity 0.33mmol / g, 30μmol): 90mg of resin was added to the solid phase synthesis tube, 2mL of DMF solution was added, and the reaction was shaken at room temperature for 15min;
[0057] (2) Fmoc deprotection: add 1 mL of 20% piperidine / DMF mixed solution to the synthesis tube, react at room temperature for 10 min, discard the solution, then add 1 mL of 20% piperidine / DMF mixed solution to the synthesis tube for 10 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com