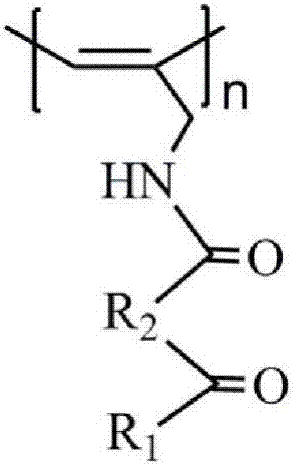
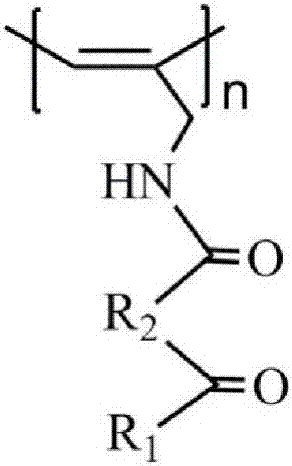
Infrared compatible microwave nano composite wave-absorbing material and preparation method thereof
A nano-composite and absorbing material technology, applied in chemical instruments and methods, other chemical processes, etc., can solve problems such as application limitations of single-function absorbing materials, and achieve unique optoelectronic characteristics, high-efficiency microwave absorption characteristics, and simple preparation technology. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0023] Step a) Add 2.48g of β-sitosterol, 0.88g of maleic anhydride, and 0.15g of 4-dimethylaminopyridine to 30mL of pyridine at 25°C, and react for 3 days under sealed and dark conditions; extract with 150mL of chloroform; The chloroform extract was obtained, dried with anhydrous sodium sulfate for 3 h, filtered, and the solvent was removed by rotary evaporation to obtain a crude product; the crude product was added to 24 mL of chloroform / petroleum ether mixture with a volume ratio of 2:1, and recrystallized twice, 50 ℃ vacuum drying for 12 hours to obtain 2.04 g of β-sitosterol maleic acid monoester;
[0024] Step b) At 25°C, dissolve 2.0g of β-sitosterol maleic acid monoester in 20mL of tetrahydrofuran, then add 0.83mL of isobutyl chloroformate, 7.0mL of N-methylmorpholine, and stir at 25°C for 15min Then add 0.198g propargylamine, react for 10h, add 25mL chloroform for extraction, obtain chloroform extract, dry with anhydrous magnesium sulfate for 3h, filter, rotary evapor...
example 2
[0030] Step a) At 25°C, add 4.01g campesterol, 3.36g citraconic anhydride, and 0.61g 4-dimethylaminopyridine to 20mL pyridine successively, and react for 8 days under sealed and dark conditions; extract with 150mL chloroform; obtain chloroform extraction solution, dried with anhydrous sodium sulfate for 5 h, filtered, and the solvent was removed by rotary evaporation to obtain a crude product; the crude product was added to 55 mL of chloroform / petroleum ether mixture with a volume ratio of 4:1, recrystallized 3 times, and vacuum-dried at 80 °C After 20h, 3.75g of campesterol citraconic acid monoester was obtained;
[0031] Step b) At 25°C, dissolve 2.74g of campesterol citraconic acid monoester in 25mL of tetrahydrofuran, then add 1.63mL of isobutyl chloroformate, 9.2mL of N-methylmorpholine, stir at 25°C for 35min, and then add 0.274 g propargylamine, reacted for 24h, added 135mL chloroform for extraction, obtained chloroform extract, dried with anhydrous magnesium sulfate fo...
example 3
[0037] Step a) At 25°C, add 4.98g brassicasterol, 2.1g citraconic anhydride, and 0.31g 4-dimethylaminopyridine to 25mL pyridine successively, and react under sealed and dark conditions for 3 days; extract with 110mL chloroform; obtain chloroform The extract was dried with anhydrous sodium sulfate for 3 hours, filtered, and the solvent was removed by rotary evaporation to obtain a crude product; the crude product was added to 45 mL of chloroform / petroleum ether mixture with a volume ratio of 2:1, recrystallized twice, and vacuum After drying for 12 hours, 4.15 g of brassicasterol citraconic acid monoester was obtained;
[0038] Step b) Dissolve 2.46g brassicasterol citraconic acid monoester in 25mL tetrahydrofuran at 25°C, then add 1.2mL isobutyl chloroformate and 8.5mL N-methylmorpholine in turn, stir at 25°C for 15min, then add 0.247g propargylamine, reacted for 10h, added 110mL chloroform for extraction, obtained chloroform extract, dried with anhydrous magnesium sulfate for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emissivity | aaaaa | aaaaa |
| emissivity | aaaaa | aaaaa |
| emissivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com