A nanobody of Glypican 3 with outstanding acid-base stability and preparation method thereof
A phosphatidylinositol and nanobody technology, applied in the biological field, can solve the problems of high cost, complex preparation process, low antibody stability, etc., and achieve the effects of low cost, simple operation and high antibody stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of embodiment 1GPC3 recombinant protein
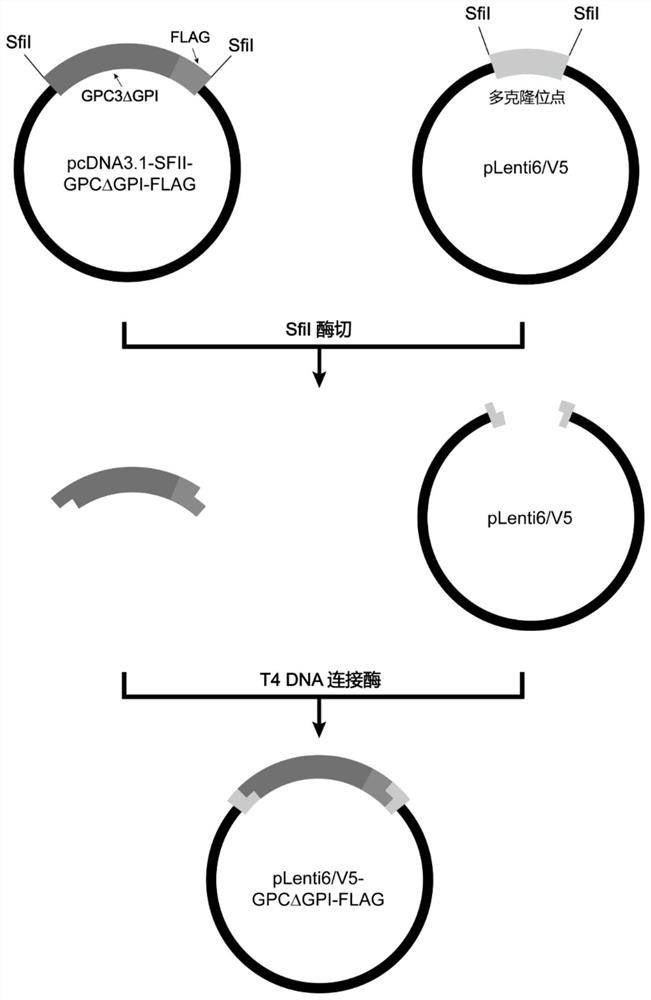
[0033] Prepare GPC3 recombinant protein with FLAG tag at the carboxy-terminus.
[0034](1) According to the coding gene sequence of GPC3 in NCBI (NCBI Reference Sequence: NM_004484.4), and based on the amino acid sequence of GPC3 in UniProt (UniProt Reference Sequence: P51654-1), the F359S mutation was performed, and the GPI region ( 1690-1740bp), the fusion sequence of GPC3ΔGPI and FLAG tag missing the GPI site was artificially synthesized (Shenzhen Huada Gene Technology Co., Ltd.), with EcoRI and NotI restriction sites at the 5' end and 3' end, respectively. The amino acid sequence encoded by GPC3ΔGPI is shown in SEQ ID NO:9 (Met Ala Gly Thr ValArg Thr Ala Cys Leu Val Val Ala Met Leu Leu Ser Leu Asp Phe Pro Gly Gln AlaGln Pro Pro Pro Pro Pro Pro Asp Ala Thr Cys His Gln Val Arg Ser Phe Phe GlnArg Leu Gln Pro Gly Leu Lys Trp Val Pro Glu Thr Pro Val Pro Gly Ser Asp LeuGln Val Cys Leu Pro Lys Gly Pro Thr Cys Cys Ser A...
Embodiment 2
[0035] Example 2 Screening of Nanobody Phage Library against GPC3
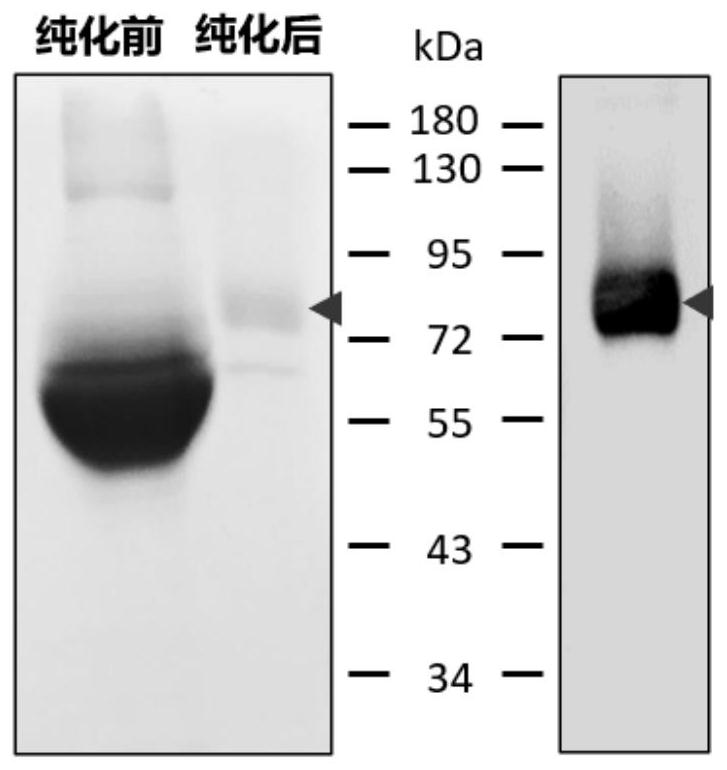
[0036] (1) Coating antigen: According to the instructions of FLAG M2 magnetic beads, the GPC3ΔGPI-FLAG protein secreted into the culture supernatant was purified to prepare GPC3ΔGPI-FLAG protein-coated magnetic beads, which were stained with Coomassie brilliant blue and immunoblotted. Test for verification. Such as figure 2 As shown, the left picture shows the Coomassie brilliant blue staining results of the culture supernatant before purification and the protein after purification. It can be seen that the size of the purified protein is between 72-85kD, and the band is single, indicating that the purity is high. The image on the right shows the results of Western blot detection using the FLAG tag antibody of the GPC3ΔGPI-FLAG recombinant protein, showing that the protein size is consistent with the Coomassie brilliant blue staining results, indicating that the purified protein is the target protein of GPC3Δ...
Embodiment 3
[0037] Preparation of Example 3 Nanobodies
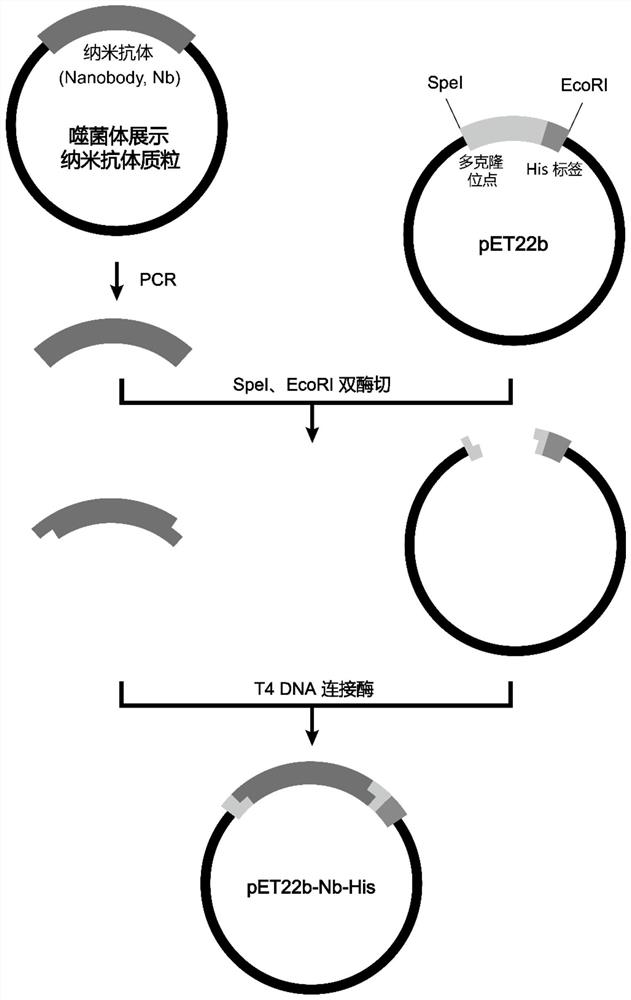
[0038] In Example 2, after completing the third round of screening for phage infection, Escherichia coli SS320 was coated on a plate, and single clones containing phage plasmids were picked for sequencing. The gene sequences of each antibody clone were analyzed and compared using Vector NTI software, and the clones with the same sequence of complementary regions CDR1, CDR2, and CDR3 were regarded as the same clone. According to the sequencing results, one of the clones with a high repetition rate was selected and labeled as G10 clone. The DNA sequence shown is shown in SEQ ID NO:8, and the encoded amino acid sequence is shown in SEQ ID NO:7. The nucleotide fragments of the selected G10 nanobody were connected to the expression vector pET22b by PCR amplification, restriction endonuclease digestion and T4 ligase connection. PCR primers for increasing the nucleotide fragment of the Nanobody: upstream primer catgactagt caagttcaat tagtc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com