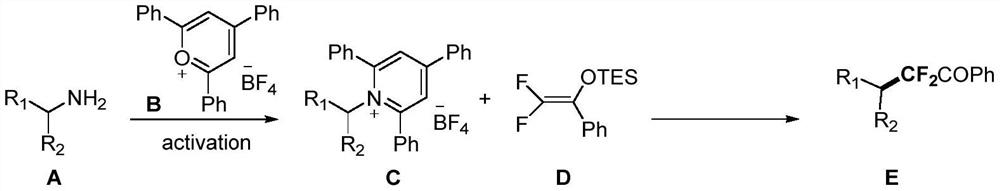
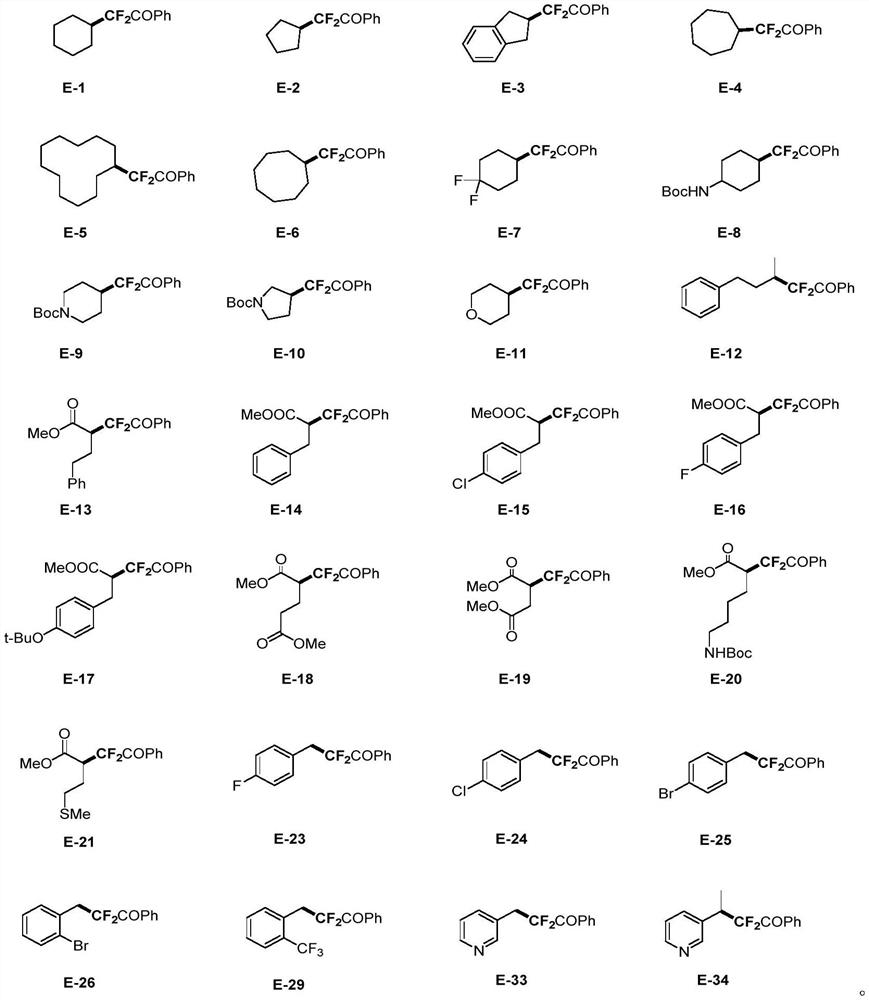
Method for difluoroalkylation after fatty amine deamination
A technology of difluoroalkyl and aliphatic amine, applied in the field of difluoroalkylation after deamination of aliphatic amine, can solve the problems of no literature report, few literature reports, etc., and achieve excellent functional group compatibility and excellent reaction diversity , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The product prepared by the preparation method of the present invention can be separated and purified by various methods, and the methods include: thin layer chromatography, column chromatography and the like. The above purification methods are conventional methods in this area. For example, when using thin-layer chromatography and column chromatography, the developing agent used can be a single solvent or a mixed solvent, such as petroleum ether or ethyl acetate-petroleum ether. mixed solvents, etc.
[0038] The above-mentioned features mentioned in the present invention, or the features mentioned in the embodiments can be combined arbitrarily. All the features disclosed in the specification of this case can be used in combination with any combination, and each feature disclosed in the specification can be replaced by any alternative feature that provides the same, equivalent or similar purpose. Therefore, unless otherwise specified, the disclosed features are only ge...
Embodiment 1-18
[0042]
[0043] Into a 25mL reaction tube, add 990.5mg (2.5mmol, 1 equivalent) of compound B, inject 2.5mL of absolute ethanol, 297.5mg (3mmol, 1.2 equivalent) of compound A-1, and heat and stir at 90°C for 5 hours to obtain compound C-1.
[0044] In the reaction tube of 25mL, add 47.7mg (0.1mmol, 1 equivalent) compound C-1, 1.8mg (2mol%) [Ir (dtbbpy) (ppy) 2 ]PF 6 , after argon replacement three times, add the corresponding solvent in the following table, inject 54.1 mg (0.2 mmol, 2 equivalents) of compound D, and under blue light irradiation, heat and stir at 50 ° C for 24 hours to obtain compound E-1, the yield of fluorine spectrum See the table below. 1 HNMR (400MHz, CDCl 3 )δ8.08(d, J=8.0Hz, 2H), 7.62(t, J=7.4Hz, 1H), 7.49(t, J=7.8Hz, 2H), 2.30–2.18(m, 1H), 1.84– 1.80(m,4H),1.70–1.66(m,1H),1.34–1.16(m,5H). 19 F NMR (376MHz, CDCl 3 )δ-108.56 (d, J=14.7Hz, 2F). Compound E-1 is a new compound.
[0045]
[0046]
Embodiment 19-21
[0048]
[0049] In a 25mL reaction tube, add 47.7mg (0.1mmol, 1 equivalent) of compound C-1, the corresponding catalyst [PC] (2.0mol%) in the table below, and add 0.25mL N,N-di Methylacetamide (DMA) and 0.75mL ethylene glycol dimethyl ether (DME), injected 54.1mg (0.2mmol, 2 equivalents) of compound D, under blue light irradiation, heated and stirred at 50°C for 24 hours to obtain compound E- 1. The yield of fluorine spectrum is shown in the table below. 1 H NMR (400MHz, CDCl 3 )δ8.08(d, J=8.0Hz, 2H), 7.62(t, J=7.4Hz, 1H), 7.49(t, J=7.8Hz, 2H), 2.30–2.18(m, 1H), 1.84– 1.80(m,4H),1.70–1.66(m,1H),1.34–1.16(m,5H). 19 F NMR (376MHz, CDCl 3 )δ-108.56 (d, J=14.7Hz, 2F). Compound E-1 is a new compound.
[0050]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com