Use of tlr agonist in combination with immune checkpoint inhibitor and vegfr inhibitor in the preparation of drugs for treating tumors
An immune checkpoint and inhibitor technology, which can be used in anti-tumor drugs, anti-animal/human immunoglobulins, drug combinations, etc., and can solve problems such as no results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
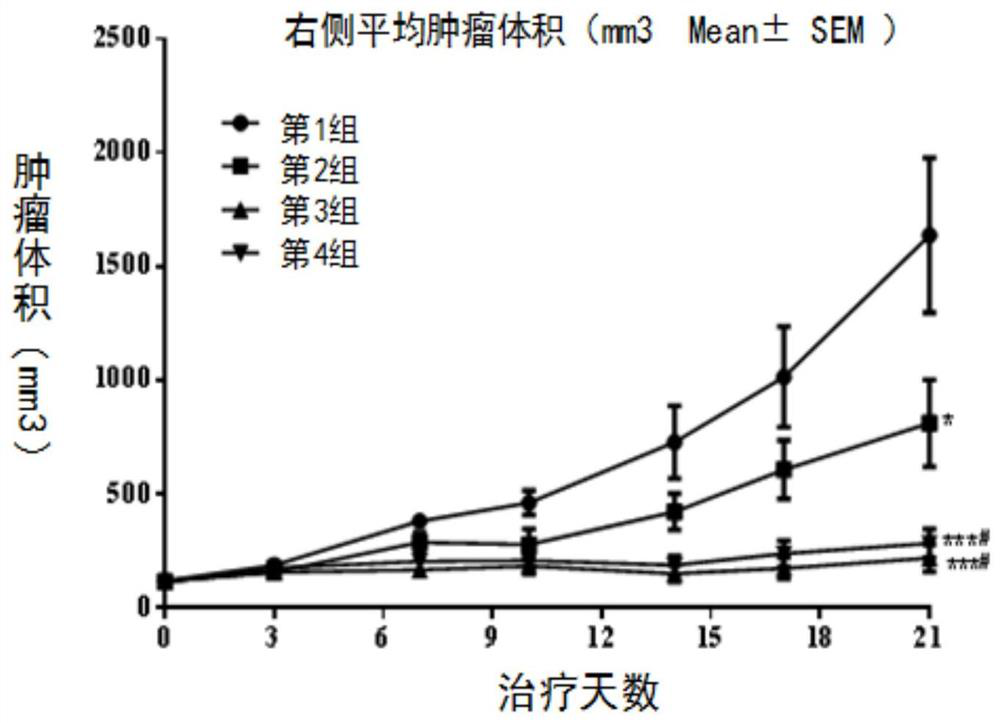
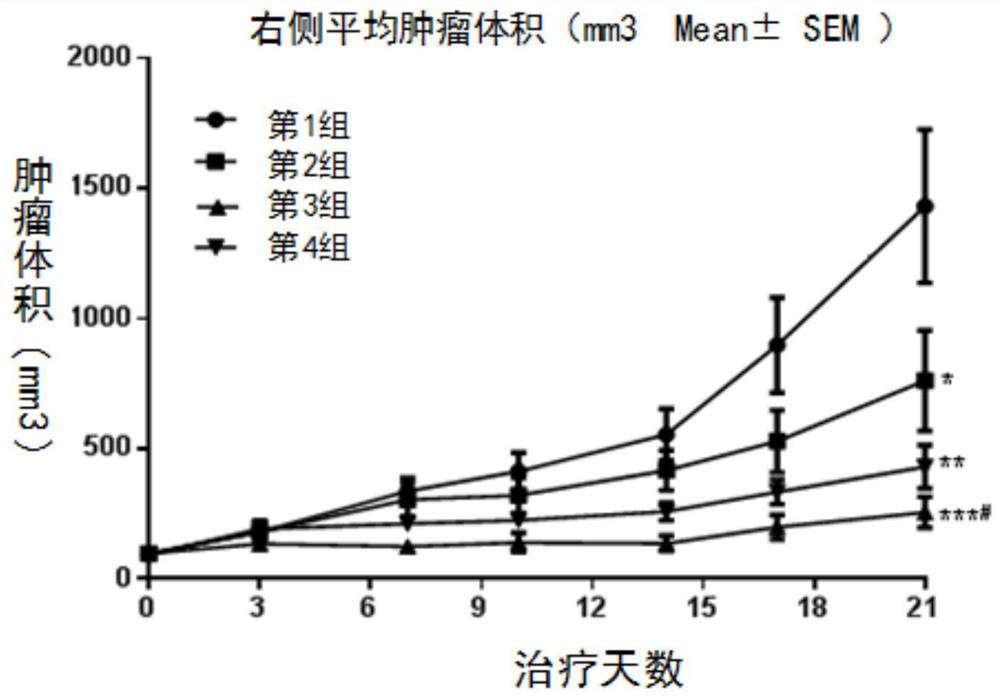
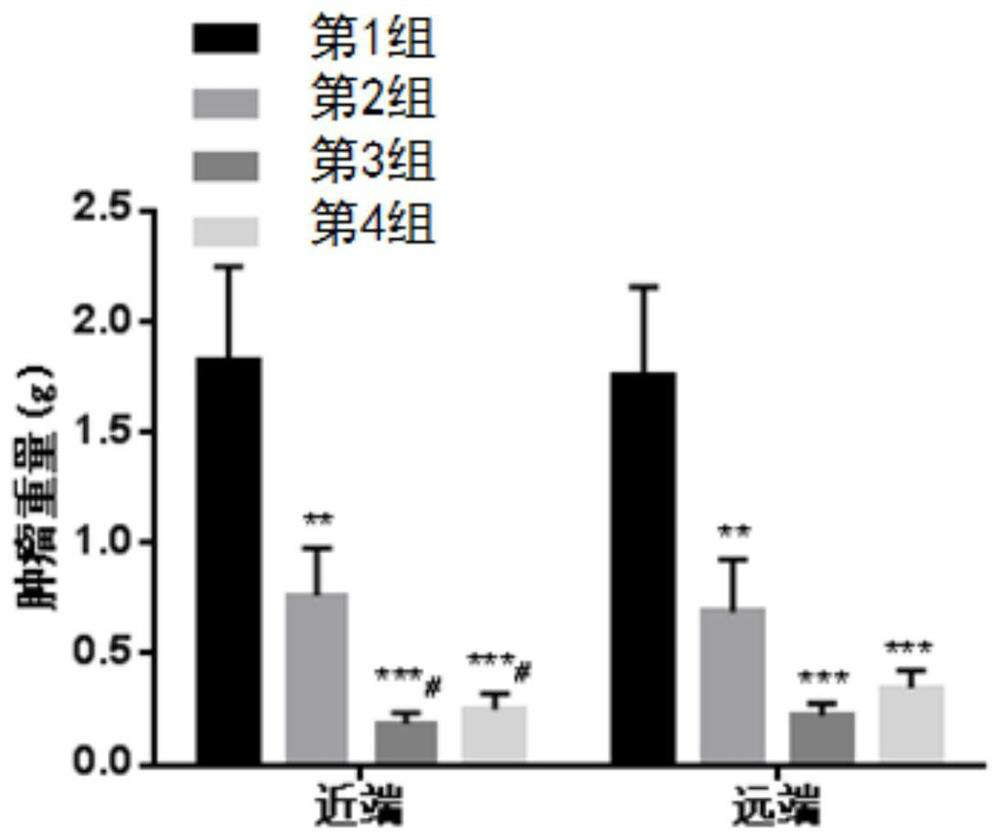
[0134] Example 1. Preclinical research Compound dihydrochloride of formula (I) (Drug A), PD-1 antibody (Drug B), and apatinib mesylate in huPD of murine colon cancer MC38 -1 Pharmacodynamic evaluation on humanized transgenic mouse animal model
[0135] 1. Experimental materials
[0136] Tumor cells: MC38 tumor cells, P7 generation was purchased from Nanjing Galaxy Bio-Pharmaceutical Co., Ltd. The cells were cultured in RPMI1640 medium containing 10% fetal bovine serum. The cells were adherent cells and were routinely digested and passaged with EDTA-containing trypsin. They were passaged twice a week and placed in a 37°C, 5% CO2 incubator for continued culture. . Tumor cells in logarithmic growth phase were used for the establishment of in vivo xenograft models.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com