Application of mesenchymal stem cells in regulation and control of monocytes of viral infection patients
A viral infection and mesenchymal stem cell technology, applied in the field of biomedical engineering, can solve the problems of delayed virus clearance, secondary infection, excessive immune activation and rapid transformation of immune suppression, etc., to achieve suppression of excessive immune response, effective treatment, and alveolar function The effect of protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] In order to enable those skilled in the art to better understand the technical solutions of the present invention, the present invention will be further described in detail below in conjunction with the accompanying drawings.
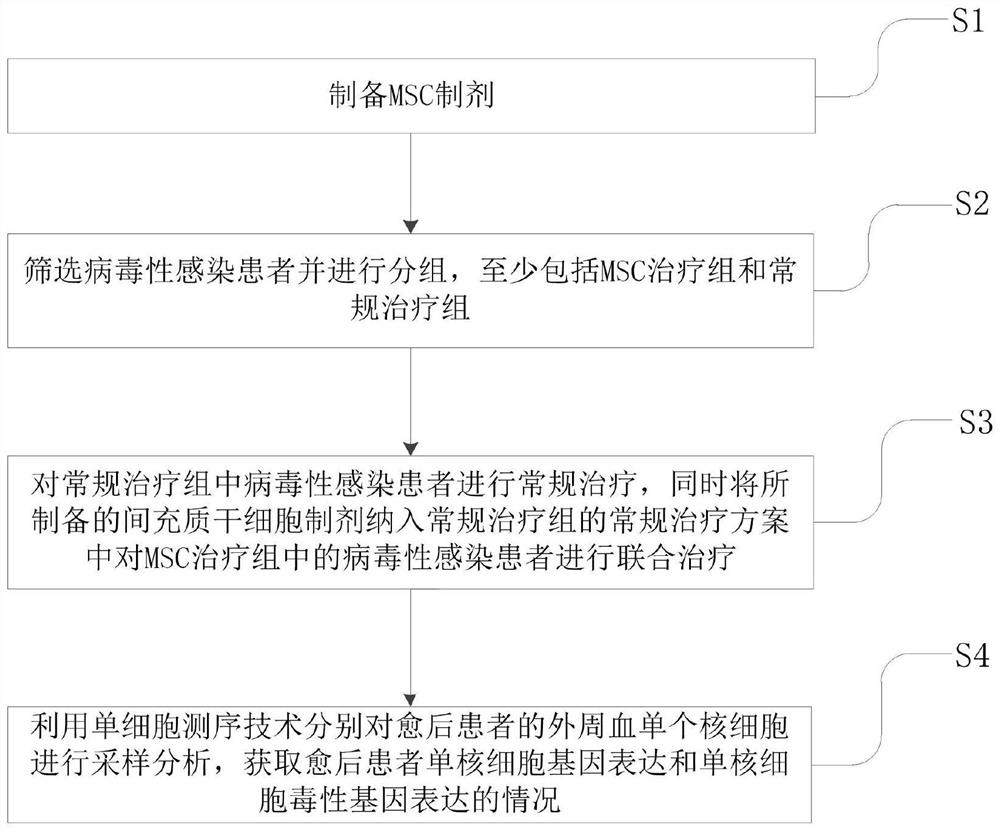
[0023] It should be noted that the conventional treatment plan mentioned in the present invention refers to the treatment plan adopted in the new coronavirus pneumonia diagnosis and treatment plan issued by the General Office of the National Health Commission and the Office of the State Administration of Traditional Chinese Medicine. MSC+ means the MSC treatment group, MSC- indicates the conventional treatment group.
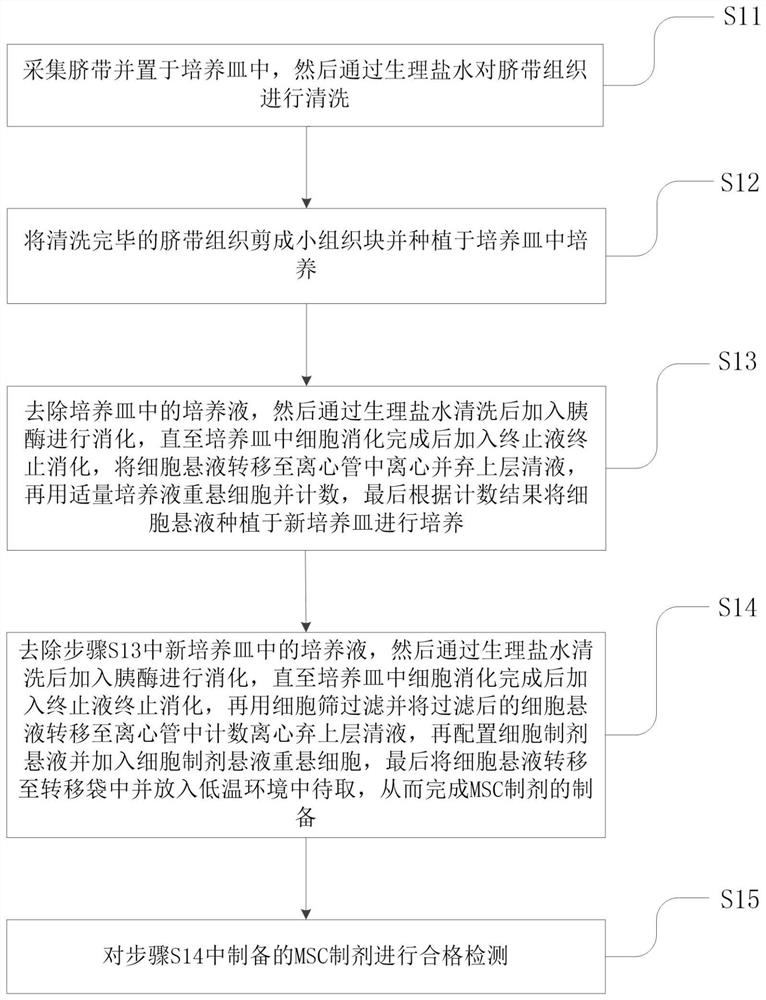
[0024] Such as figure 1 As shown, a kind of mesenchymal stem cells (mesenchymal stem cells, MSC) is used to regulate the application of monocytes in patients with viral infection, by applying mesenchymal stem cells to regulate monocyte gene expression and regulate monocyte toxicity genes Expression to achieve effective treatment o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com