In-situ stable overexpression method of glycoprotein MUC16
An overexpression and glycoprotein technology, applied in the field of biomedicine, can solve the problem of unsuitable accommodation of target genes, and achieve the effect of long-term stable overexpression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1. Basic information of vector system and sgRNA design:
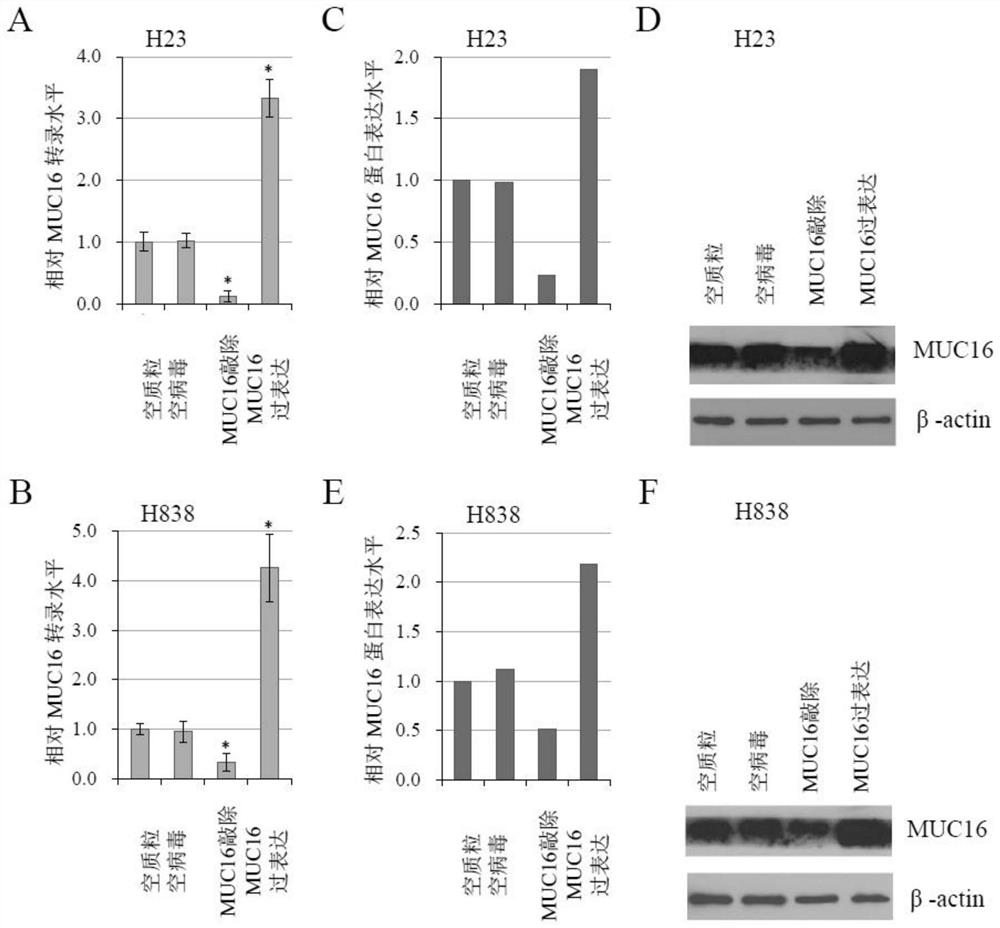
[0038]MUC16 was overexpressed using the Lenti-CRISPR-dCas9 system, and three sgRNAs were used to increase the activation efficiency. The vector construction and lentiviral packaging followed the reference (Konermann S, Brigham MD, Trevino AE, etal. Genome-scale transcriptional activation by an engineered CRISPR-Cas9complex.Nature 2015;517:583–588), the Lenti-CRISPR-dCas9 system comes from Zhang Feng’s laboratory. sgRNA sequences were designed using CRISPRdirect.
[0039] Table 1. Lenti-CRISPR-dCas9 system used for MUC16 overexpression
[0040] Gene Catalog# Plasmidname MUC16overexpression Plasmid#61425 lentidCAS-VP64_Blast Plasmid#61426 lentiMS2-P65-HSF1_Hygro Plasmid#61427 lentisgRNA(MS2)_zeobackbone
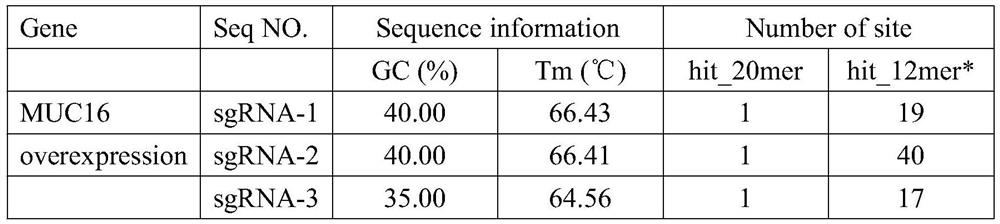
[0041] Table 2. MUC16 overexpression sgRNA
[0042] Gene Seq NO. sgRNA sequence MUC16overexpression sgRNA-1 GGTTTCTAAGACATCACACA sgRNA-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com