Kit for clinically detecting active urokinase receptor in plasma of new coronal pneumonia patient
A technology of urokinase receptor and kit, which is applied in the field of medical detection and can solve the problem of low expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
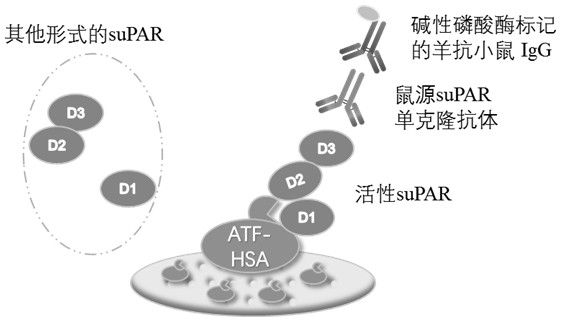
[0021] Example 1 Preparation of an ELISA detection kit pre-coated with a fusion protein that can bind to active suPAR
[0022] (1) Prepare washing solution / sample diluent
[0023] The washing solution and sample diluent contained 0.05% Tween 20 in TBS buffer, and the formula of TBS was 20 mM Tris-HCl, 150 mM NaCl, 2.7 mM KCl.
[0024] (2) Configure the coating solution
[0025] The coating solution is 50 mM bicarbonate buffer solution (pH 9.6), specifically 1.59 gNaCO in 1L buffer solution 3 ,, 2.93 g NaHCO 3 .
[0026] (3) Prepare blocking solution
[0027] The blocking solution was prepared from TBST, pH 7.4 buffer solution containing 4% sucrose and 3% BSA.
[0028] (4) Preparation of substrate chromogenic solution
[0029] The substrate chromogenic solution is 0.1 M Tris-HCl, 0.1 M NaCl, 5 mM MgCl and 1 mg / ml PNPP.
[0030] (5) Recombinant expression and purification of suPAR standard
[0031] The recombinant suPAR was expressed in Drosophila embryonic cell S2 and p...
Embodiment 2
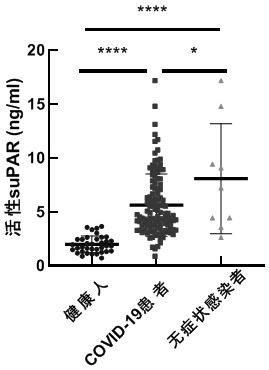
[0038] Example 2 Using an ELISA detection kit to detect the level of active soluble urokinase receptor (suPAR) in COVID-19 patients, asymptomatic infected persons or healthy individuals
[0039] 1. Add 100 μl of standard substances of different concentrations to the microplate, and then add the diluted plasma sample of the patient to be tested (diluted 5 times with the sample diluent) to the other wells of the microtiter plate. Each plasma sample has 2 replicates. The 96-well plate was sealed with sealing tape to prevent liquid evaporation, and incubated at 37°C for 2h. Uncover the sealing tape, dry the liquid in the wells of the microplate reader, and absorb the washing solution with a row gun, wash each well 5 times, after the last wash, pat the microplate dry on absorbent paper and ensure that the wells are completely dry. No air bubbles remained.
[0040] 2. Add the rabbit-derived anti-active suPAR polyclonal antibody diluted 200-800 times with the sample diluent to the E...
Embodiment 3
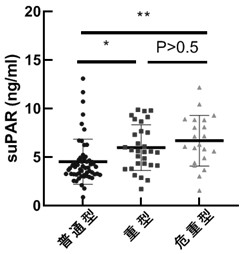
[0044] Example 3 Active suPAR levels in plasma of COVID-19 patients (common type, severe type and critical type)
[0045] The COVID-19 patients in Example 2 were classified in more detail into common type, severe type and critical type, and then the levels of active suPAR in these three groups were analyzed, and it was found that the level of suPAR in the blood of patients with severe disease The higher the result, see image 3 . The average plasma concentrations of normal, severe and critical patients were 4.57 ng / ml, 5.97 ng / ml and 6.68 ng / ml, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com