Application of sakuranetin in hair loss prevention and hair growth
A technology of sakura element and hair loss, applied in the field of medicine, compound and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
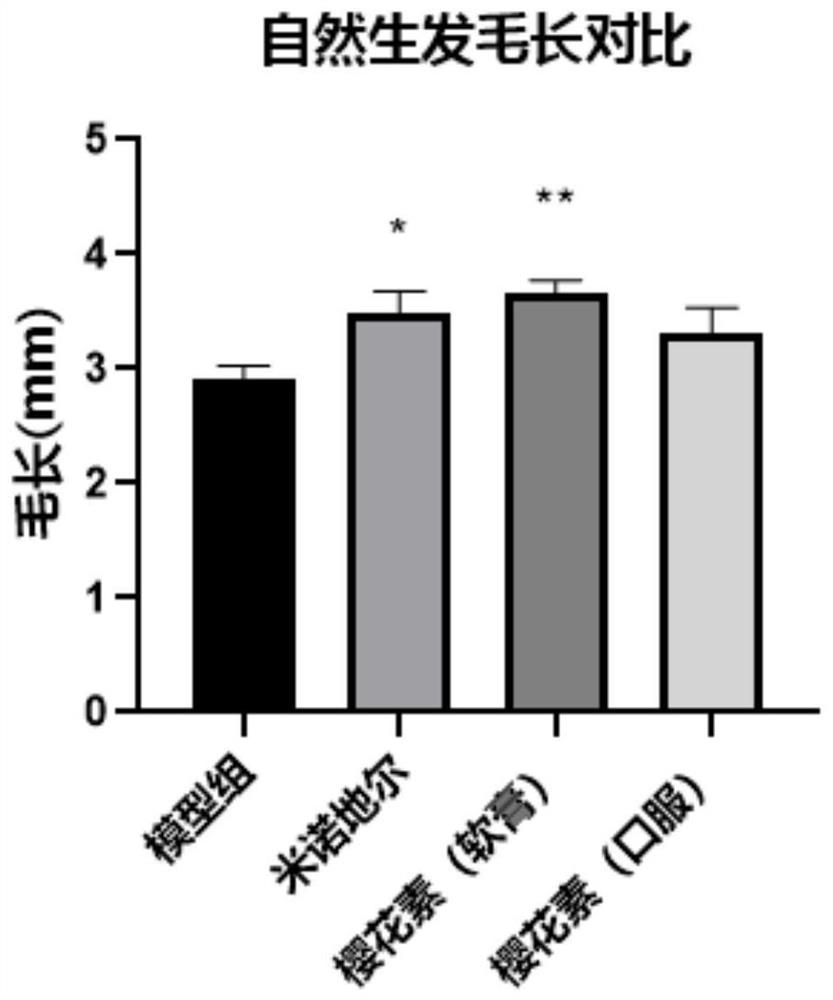
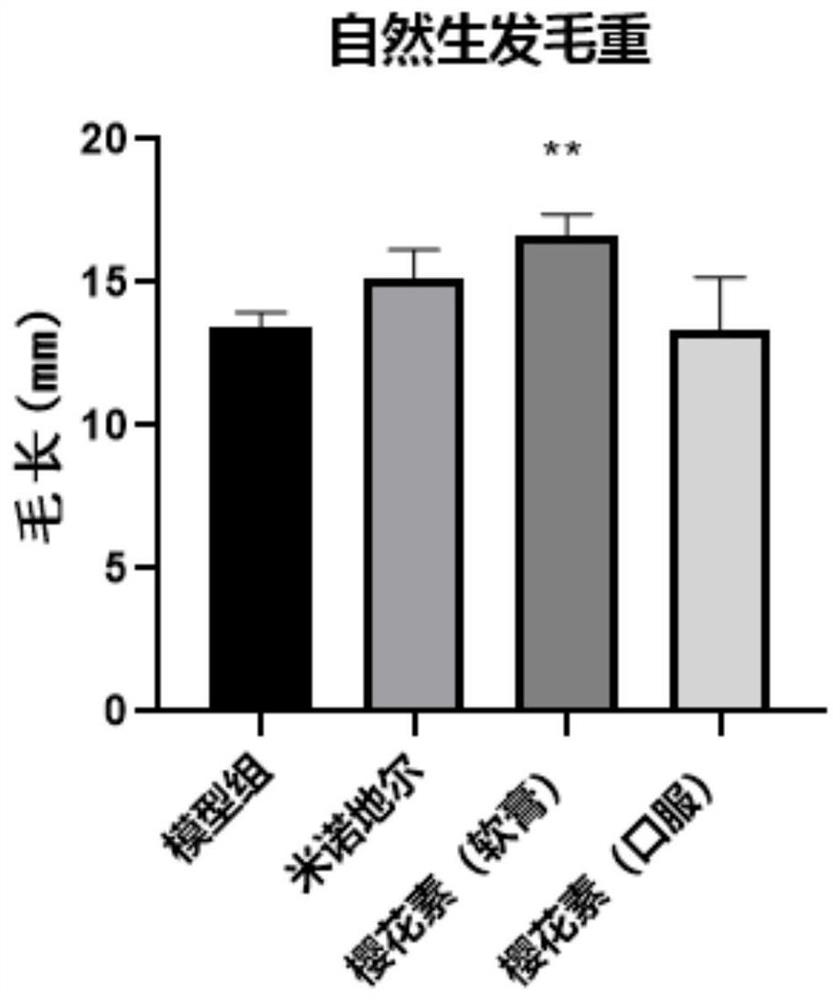
[0038] Example 1: Curative effect evaluation experiment of sakura element on natural hair growth model of C57BL / 6 mice
[0039] 1. Experimental animals
[0040] The experimental animals were male C57BL / J6, 8 weeks old, weighing 18-20 g, purchased from the Comparative Medical Center of Yangzhou University. C57BL / 6 mice were reared under normal conditions, temperature 25±2°C, humidity 50%-70%, light and dark alternately for 12 hours, free to eat and drink, and change the corncob litter every two days until they adapt The experiments were started after 7 days in the rearing environment.
[0041] 2. Drug configuration
[0042] 4% chloral hydrate: 0.4g of chloral hydrate was dissolved in 10ml of normal saline, vortex centrifuged until completely dissolved, and prepared into 4% chloral hydrate solution.
[0043] 2.1 2.5% (O / W) sakurain cream for external use, made from the following raw materials:
[0044]400 parts by weight of sakurain, 1000 parts by weight of stearic acid, 200...
Embodiment 2
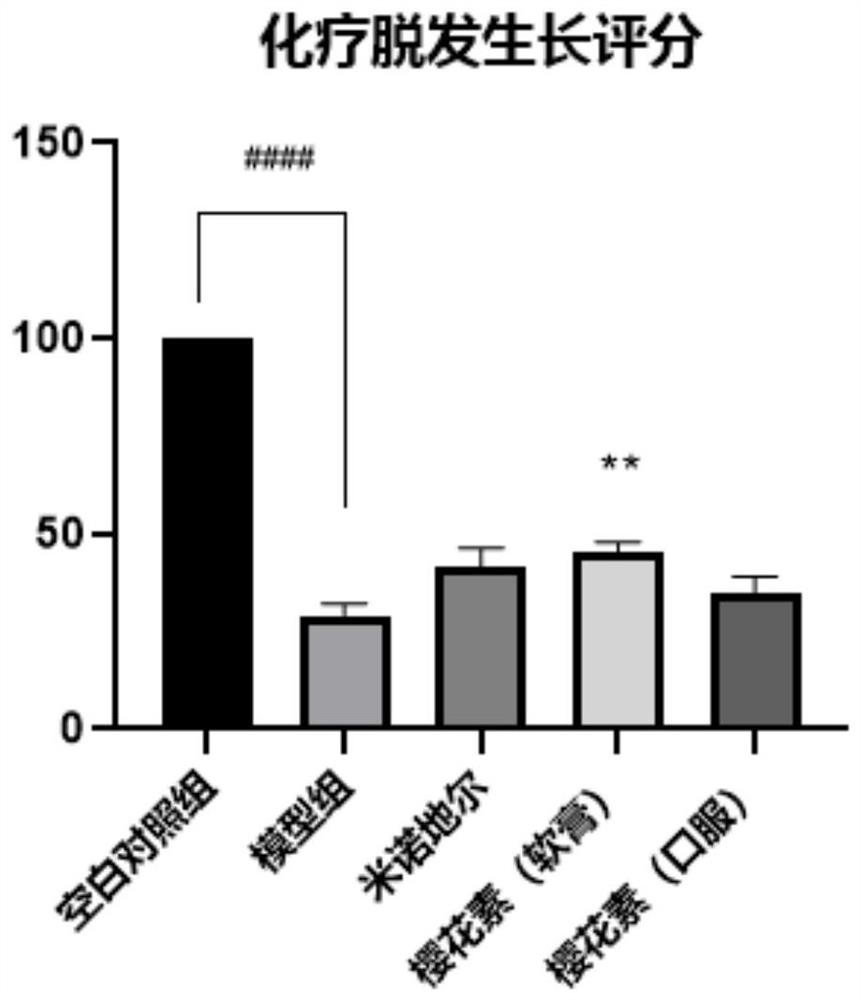
[0058] Example 2: Curative effect evaluation experiment of sakura element on C57BL / 6 mouse chemotherapy hair loss model
[0059] 1. Experimental animals
[0060] The experimental animals were male C57BL / J6, 8 weeks old, weighing 18-20 g, purchased from the Comparative Medical Center of Yangzhou University. C57BL / 6 mice were reared under normal conditions, temperature 25±2°C, humidity 50%-70%, light and dark alternately for 12 hours, free to eat and drink, change corncob bedding every two days, and wait for them to adapt The experiments were started after 7 days in the rearing environment.
[0061] 2. Drug configuration
[0062] The configuration of 4% chloral hydrate is the same as above.
[0063] The configuration of the cherry blossom ointment is the same as above.
[0064] The configuration of sakurain oral suspension is the same as above.
[0065] Cyclophosphamide for injection: Dissolve 0.2g cyclophosphamide in 20ml normal saline, vortex centrifuge until completely d...
Embodiment 3
[0078] Example 3: Curative Effect Evaluation Experiment of Cherry Blossom on C57BL / 6 Mouse Androgenetic Alopecia Model 1. Experimental Animals
[0079] The experimental animal is C57BL / J6, 8 weeks old, weighing 18-20g, purchased from the Comparative Medical Center of Yangzhou University. C57BL / 6 mice were reared under normal conditions, temperature 25±2°C, humidity 50%-70%, light and dark alternately for 12 hours, free to eat and drink, change corncob bedding every two days, and wait for them to adapt The experiments were started after 7 days in the rearing environment.
[0080] 2. Drug configuration
[0081] The configuration of 4% chloral hydrate is the same as above.
[0082] The configuration of the cherry blossom ointment is the same as above.
[0083] The configuration of sakurain oral suspension is the same as above.
[0084] Testosterone propionate injection (1mL: 25mg, Guangzhou Baiyunshan Mingxing Pharmaceutical Co., Ltd.), diluted with edible oil to 1mg / ml. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com