Application of rebamipide to alopecia prevention and hair growth
A technique for rebamipide and hair loss, which is applied in the field of compounds and their preparations, and medicines, and can solve problems such as rebamipide that have not yet been publicly reported in the literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
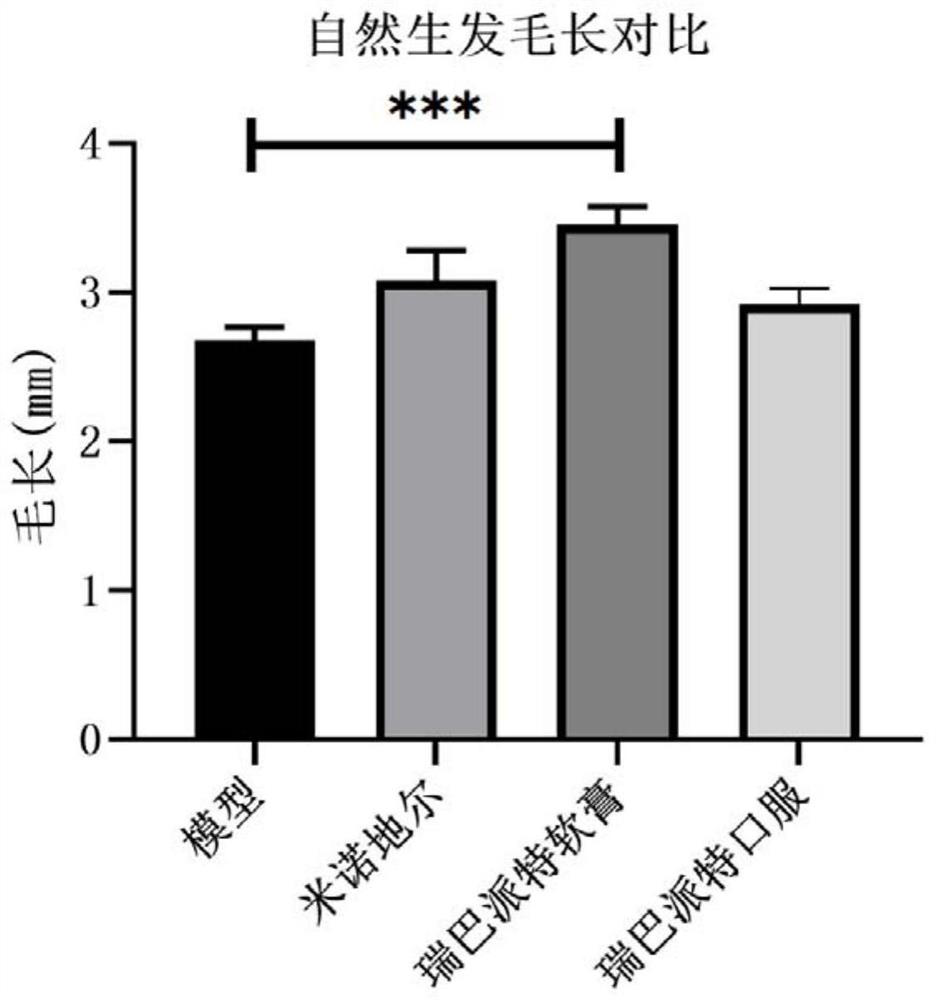
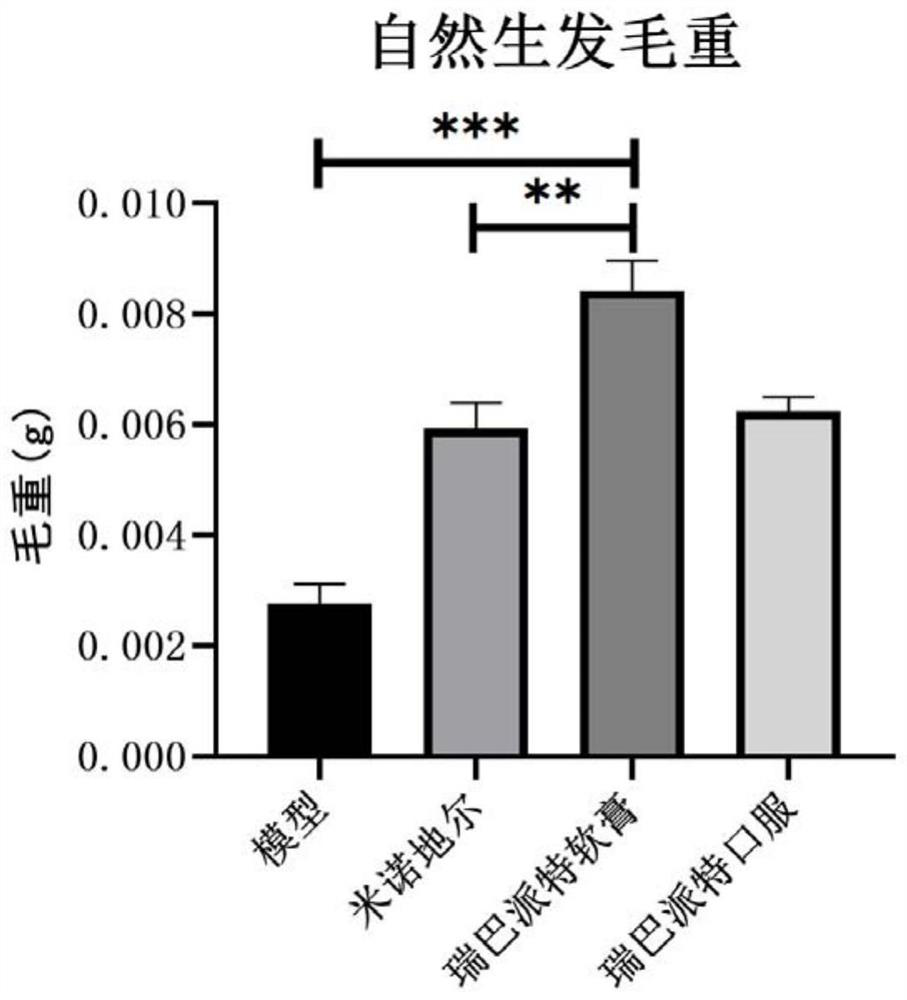
[0038] Example 1: The curative effect evaluation experiment of rebamipide on the natural hair growth model of C57BL / 6 mice
[0039] 1. Experimental animals
[0040]The experimental animals were male C57BL / J6, 8 weeks old, weighing 18-20 g, purchased from the Comparative Medical Center of Yangzhou University. C57BL / 6 mice were reared under normal conditions, temperature 25±2°C, humidity 50%-70%, light and dark alternately for 12 hours, free to eat and drink, change corncob bedding every two days, and wait for them to adapt The experiments were started after 7 days in the rearing environment.
[0041] 2. Drug configuration
[0042] 4% chloral hydrate: 0.4g of chloral hydrate was dissolved in 10ml of normal saline, vortex centrifuged until completely dissolved, and prepared into 4% chloral hydrate solution.
[0043] 2.1 4% (O / W) rebamipide cream for external use, made from the following raw materials:
[0044] 400 parts by weight of rebamipide, 1000 parts by weight of stearic...
Embodiment 2
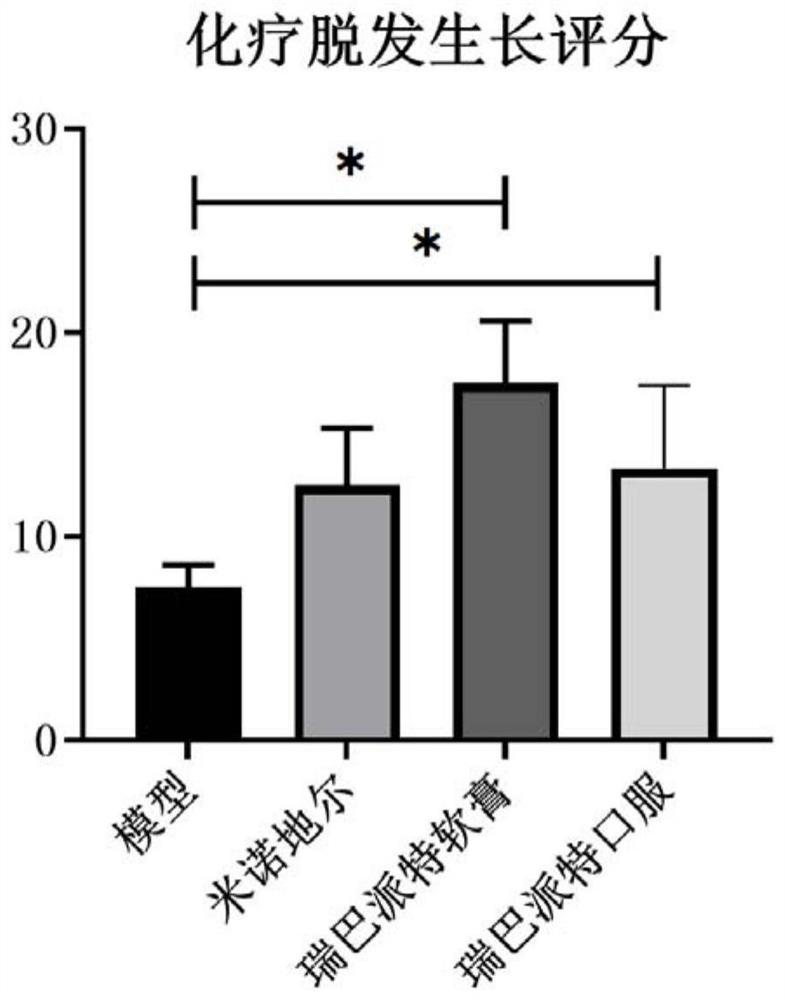
[0058] Example 2: The curative effect evaluation experiment of rebamipide on C57BL / 6 mouse chemotherapy alopecia model
[0059] 1. Experimental animals
[0060] The experimental animals were male C57BL / J6, 8 weeks old, weighing 18-20 g, purchased from the Comparative Medical Center of Yangzhou University. C57BL / 6 mice were reared under normal conditions, temperature 25±2°C, humidity 50%-70%, light and dark alternately for 12 hours, free to eat and drink, change corncob bedding every two days, and wait for them to adapt The experiments were started after 7 days in the rearing environment.
[0061] 2. Drug configuration
[0062] The configuration of 4% chloral hydrate is the same as above.
[0063] The configuration of rebamipide ointment is the same as above.
[0064] The configuration of rebamipide oral suspension is the same as above.
[0065] Cyclophosphamide for injection: Dissolve 0.2g cyclophosphamide in 20ml normal saline, vortex centrifuge until completely dissolve...
Embodiment 3
[0078] Example 3: The curative effect evaluation experiment of rebamipide on C57BL / 6 mouse androgenetic alopecia model
[0079] 1. Experimental animals
[0080] The experimental animal is C57BL / J6, 8 weeks old, weighing 18-20g, purchased from the Comparative Medical Center of Yangzhou University. C57BL / 6 mice were reared under normal conditions, temperature 25±2°C, humidity 50%-70%, light and dark alternately for 12 hours, free to eat and drink, change corncob bedding every two days, and wait for them to adapt The experiments were started after 7 days in the rearing environment.
[0081] 2. Drug configuration
[0082] The configuration of 4% chloral hydrate is the same as above.
[0083] The configuration of rebamipide ointment is the same as above.
[0084] The configuration of rebamipide oral suspension is the same as above.
[0085] Testosterone propionate injection (1mL: 25mg, Guangzhou Baiyunshan Mingxing Pharmaceutical Co., Ltd.), diluted with edible oil to 1mg / ml. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com