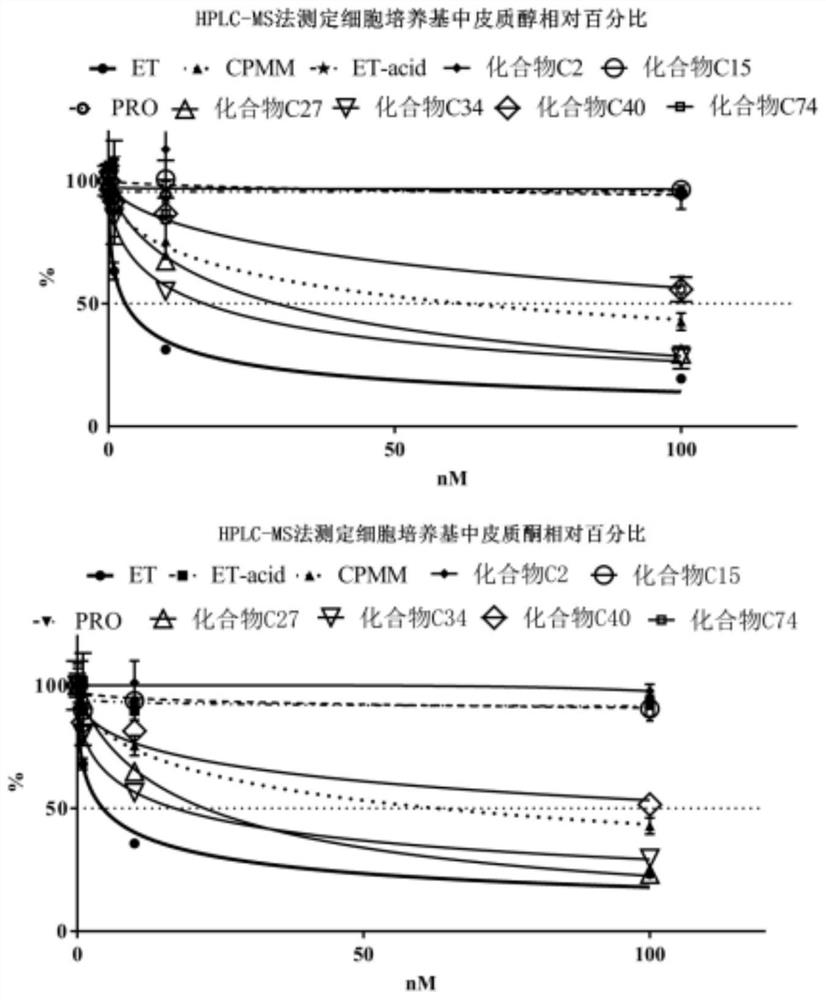
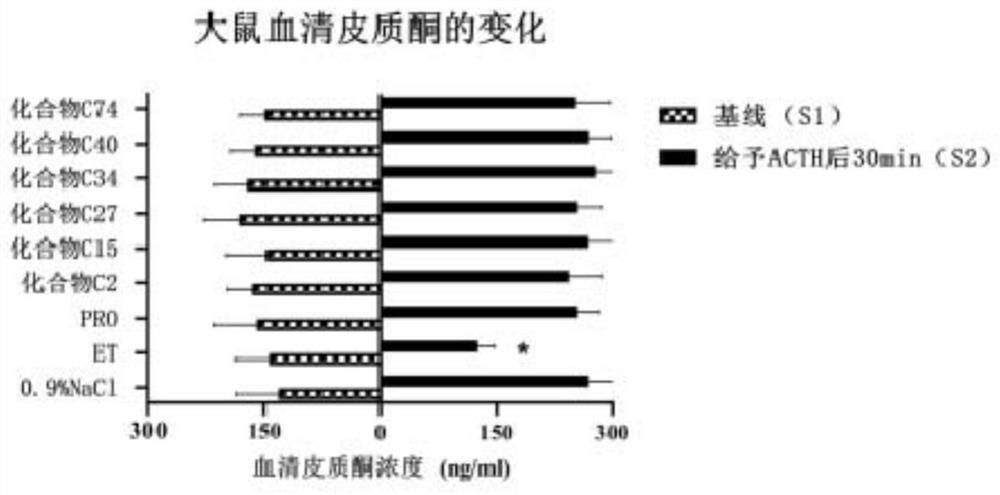
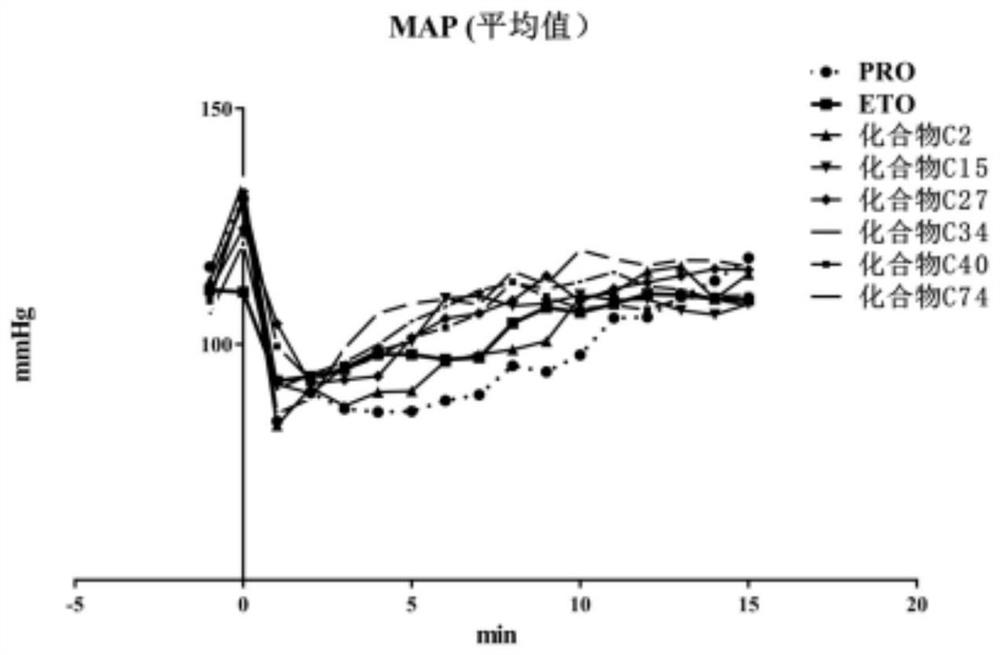
Keto-substituted heterocyclic compounds and their anesthetic effects
A compound, unsubstituted technology, used in anesthetics, drug combinations, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111]
[0113]
[0115]
[0117]
[0119]
[0121]
[0122] At room temperature, compound A-2 (3.5 g, 16.2 mmol), N,O-dimethylhydroxylamine
[0124]
[0127]
[0130]
[0132] At room temperature, the PTSA.H
[0133]
[0135]
[0137] Compound C2: 91 mg, ESI [M+H]
[0138]
[0139] Compound C3: 39 mg, ESI [M+H]
[0140]
[0142]
[0144]
[0146]
[0147] Compound C7: 46 mg, ESI [M+H]
[0148]
Embodiment 2
[0150]
[0152]
Embodiment 3
[0154]
[0156]
[0159]
[0161]
[0163]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com