Target genome for gene detection of non-small cell lung cancer patient and related evaluation method, application and kit
A non-small cell lung cancer and gene detection technology, applied in the field of bioinformatics, can solve problems such as expensive, unsatisfactory, and missing the best treatment time, and achieve the effect of avoiding economic burden and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] In this embodiment 1, a solid tumor is used as a sample to be tested, and the prediction of its tumor mutation load is taken as an example for illustration.
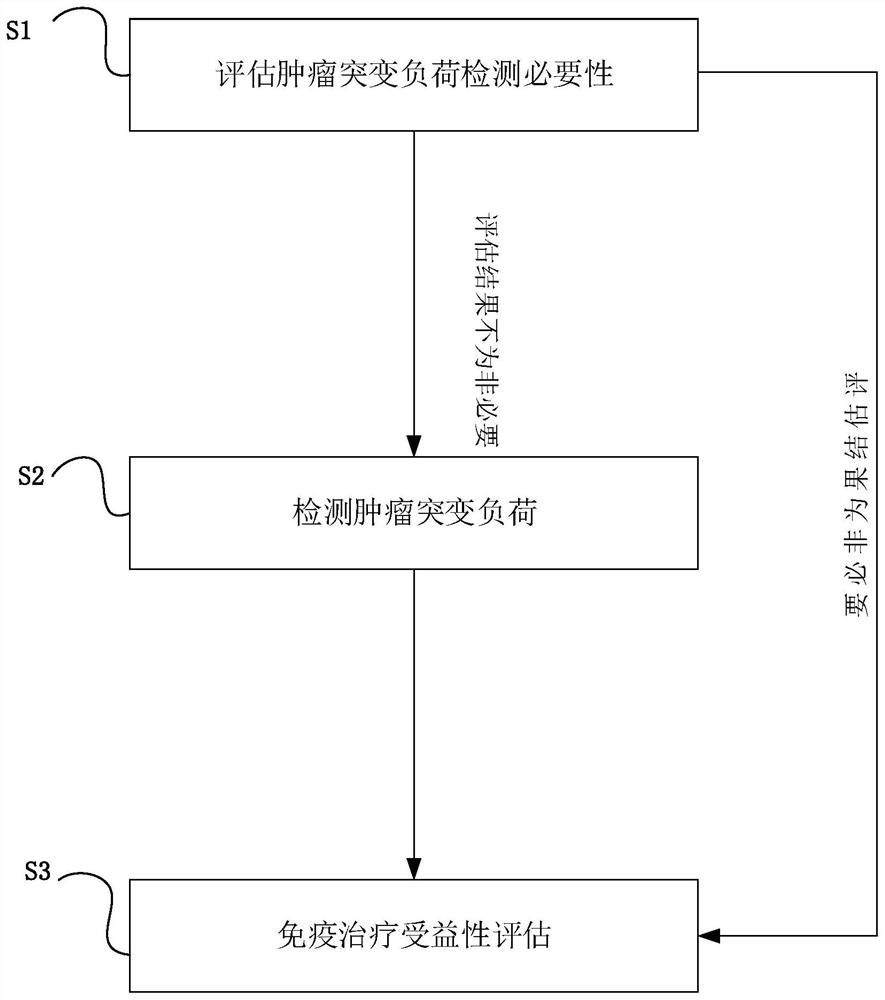
[0027] figure 1 It is a flow chart of the method for evaluating the benefit of immunotherapy involved in Example 1 of the present invention.
[0028] like figure 1 As shown, this embodiment 1 provides a method for evaluating the benefit of immunotherapy, the method comprising the following steps:
[0029] Step 1 (S1), assess the necessity of tumor mutation burden detection, specifically:
[0030] Evaluation of the necessity of tumor mutation burden detection in patients with non-small cell lung cancer (NSCLC) based on target genome.
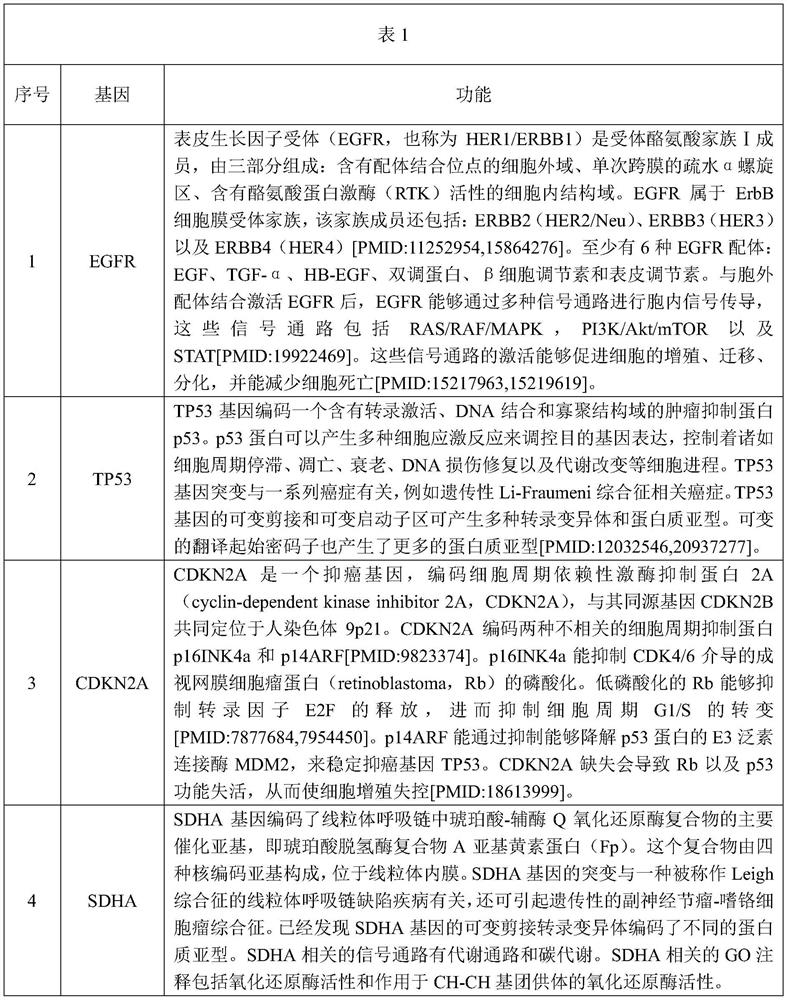
[0031] The genes included in the selected target genome and corresponding descriptions are shown in Table 1.
[0032]
[0033] Design corresponding probes for the target genome, and then based on the designed probes, perform capture sequencing on a sample from a patient with n...
Embodiment 2
[0063] This Example 2 is to demonstrate that the target genome in Example 1 can be used in the assessment of the necessity of tumor mutation burden detection and the assessment of the benefit of immunotherapy in patients with non-small cell lung cancer.
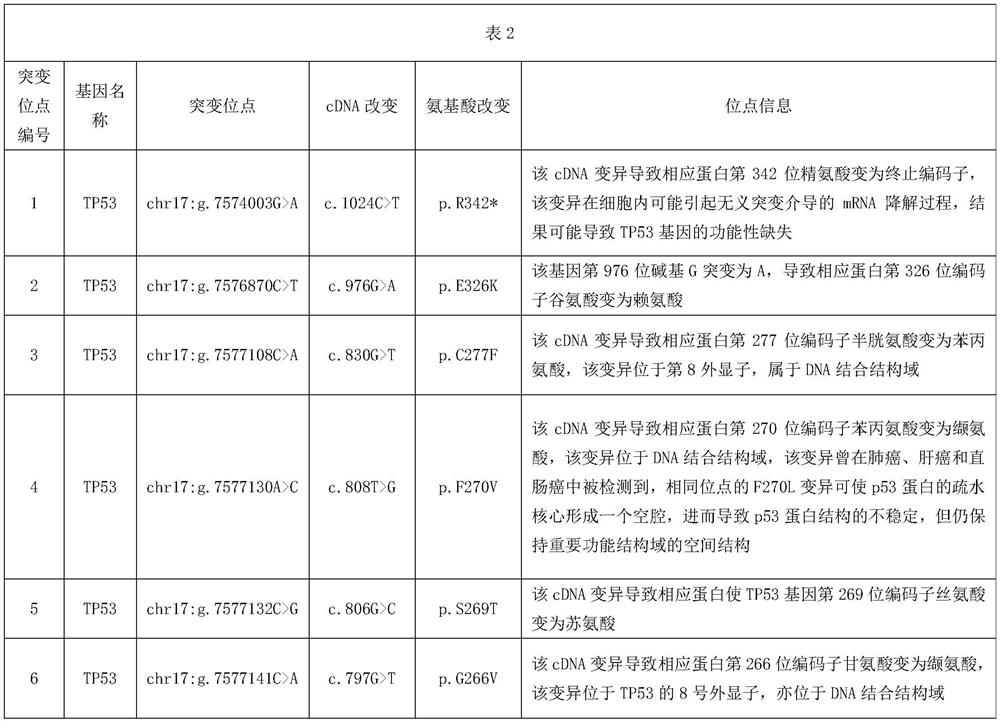
[0064] The verification of Example 2 is based on the sample source information shown in Table 2.
[0065]
[0066] In this example:
[0067] The first step is to use the above-mentioned large panel method to sequence the 291 samples and detect TMB: by constructing a sample DNA sequencing library, and using specific probes to capture and enrich the target region of the library, this process is carried out using a kit ; The captured library can realize one-time detection of multiple mutations of multiple genes through high-throughput sequencing.
[0068] Specifically as follows:
[0069] (1) Using DNA extracted from formalin-fixed paraffin-embedded (FFPE) and blood samples (paired samples) as materials, it was fragmented, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com