Nilotinib composition with improved solubility
A technology of nilotinib and composition, which is applied in the directions of drug combination, organic active ingredients, medical preparations of non-active ingredients, etc., can solve problems such as poor water solubility and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
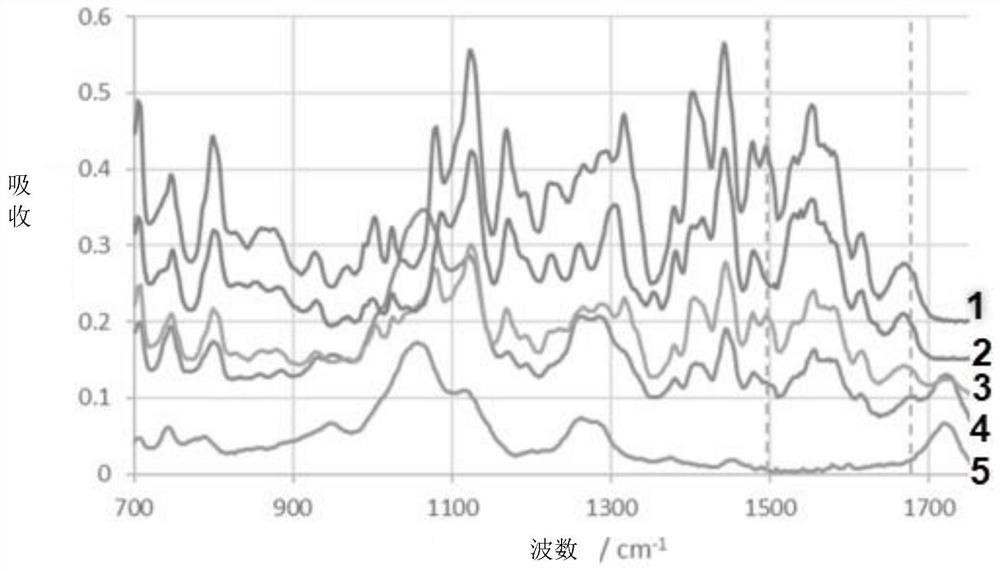
Image
Examples
Embodiment 1
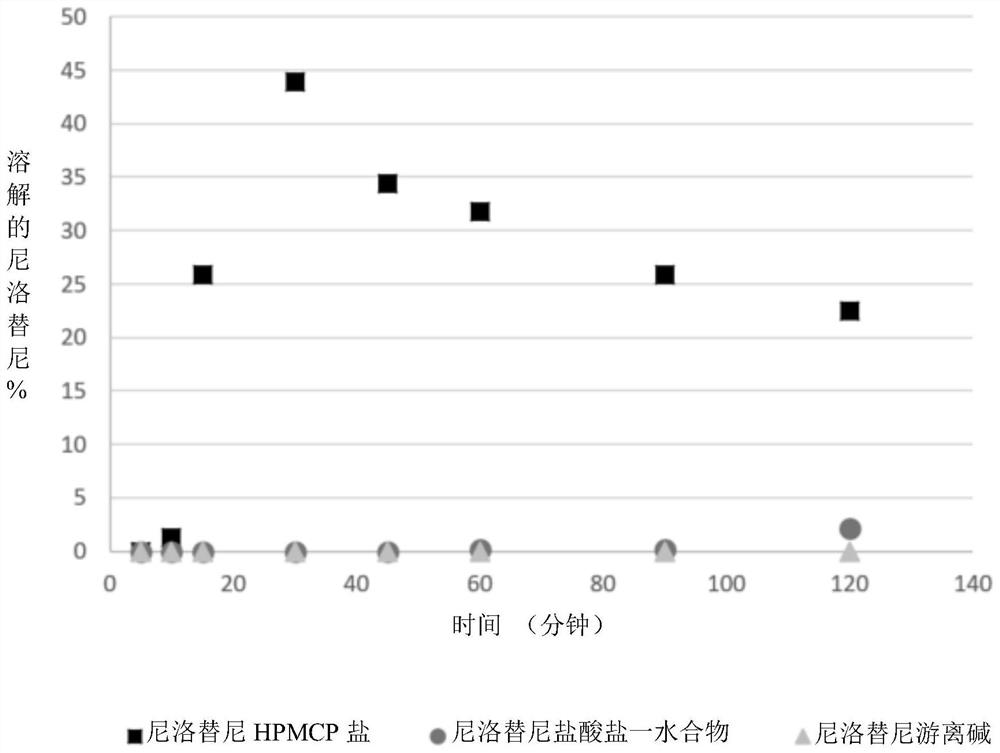
[0126] Preparation and Analysis of Embodiment 1 Nilotinib HPMCP Salt
[0127] Nilotinib: The preparation of HPMCP salt is as follows: Nilotinib free base and HPMCP are added in the mixed solvent of methanol and dichloromethane (1:1 volume ratio) to form a solution, basic Nilotinib and acidic polymer HPMCP form ionic bonds. 1.2 g of HPMCP was dissolved in a certain volume of solvent by magnetic stirring, then 0.8 g of nilotinib free base was added and allowed to dissolve. Transfer the solution to a 100mL volumetric flask, and add a certain volume of solvent to constant volume.
[0128] The resulting nilotinib HPMCP salt containing 40% nilotinib (mass ratio) was separated by spray drying with a Buchi miniature spray dryer B290 (BüCHI Labortechnik AG, Switzerland) equipped with an inert cycle B295. A high-performance cyclone separator is used for separation, and the 50mL blue cap flask can be directly mounted to the cyclone separator for product collection. The parameter setti...
Embodiment 2
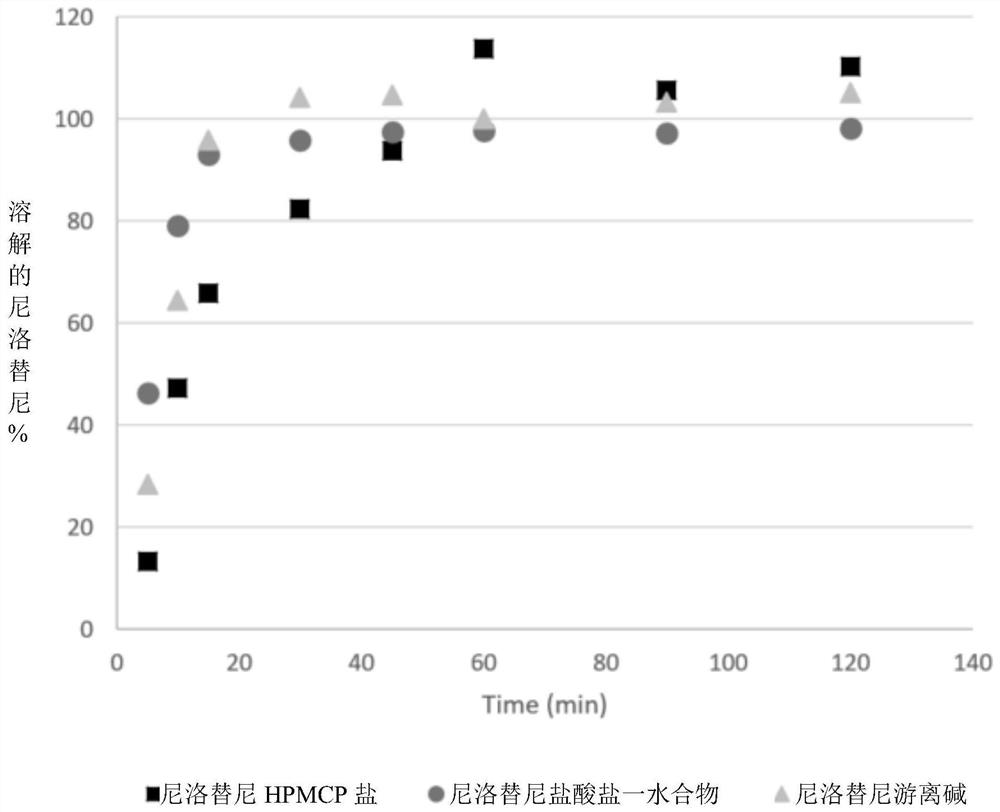
[0146] Embodiment 2 in vivo test
[0147] The prepared nilotinib HPMCP salt composition was tested in vivo in rats: young Sprague-Durer rats (generally n=4-6) fasted the day before were treated with fasted or fed state (fasting state: from 12 hours before the medication to 4 hours after the medication is not allowed to take food) test or control.
[0148] Test compositions and controls were administered orally by gavage through PE205 tubing attached to a syringe with water or 0.5% HPMC to aid wetting and prevent clumping. Rats were returned to the IVC cages and had normal access to water. Alternatively, capsules or tablets may also be administered. The test and control formulations were identical except for the presence or absence of polymer. Alternatively, the control may consist of drug only.
[0149] Blood samples were taken from the neck of the rats at 0.5, 1, 2, 6, 24 and 48 hours (sometimes 12 hours) post-injection using 1 mL disposable syringes and 20 gauge needles....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com