Novel application of selepravastatin
A technology with a new use and route of administration, applied in the new use field of selepravatide, which can solve the problems of poor safety and easy recurrence of membranous nephropathy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
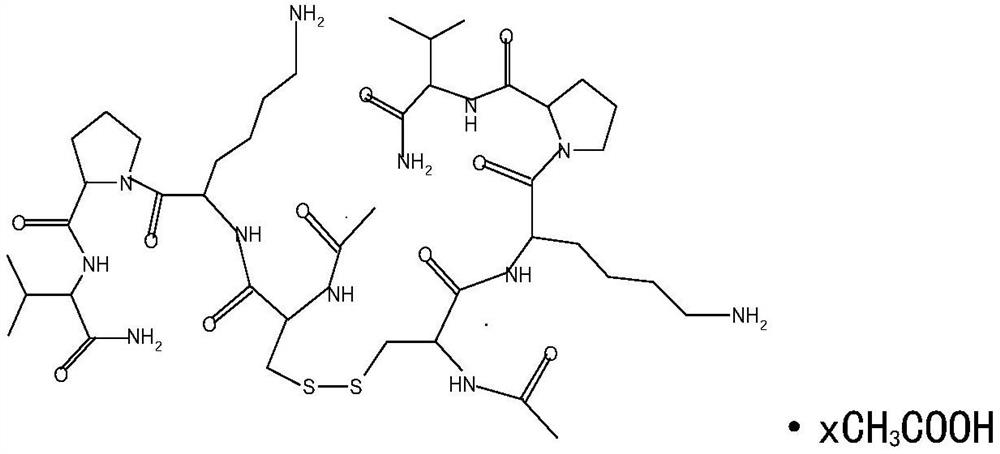
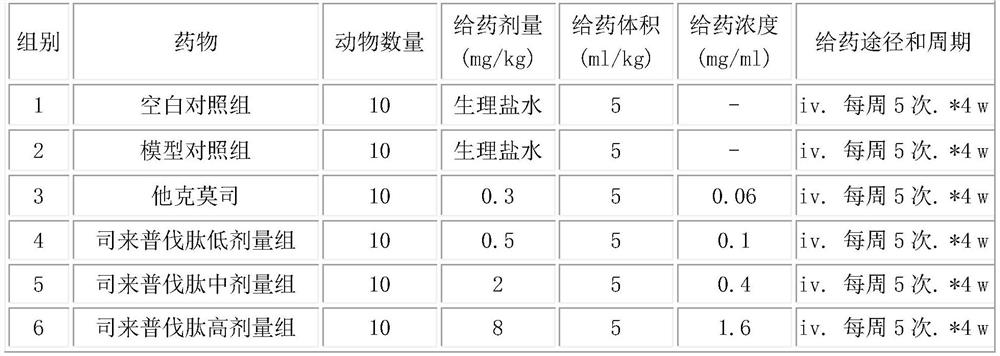
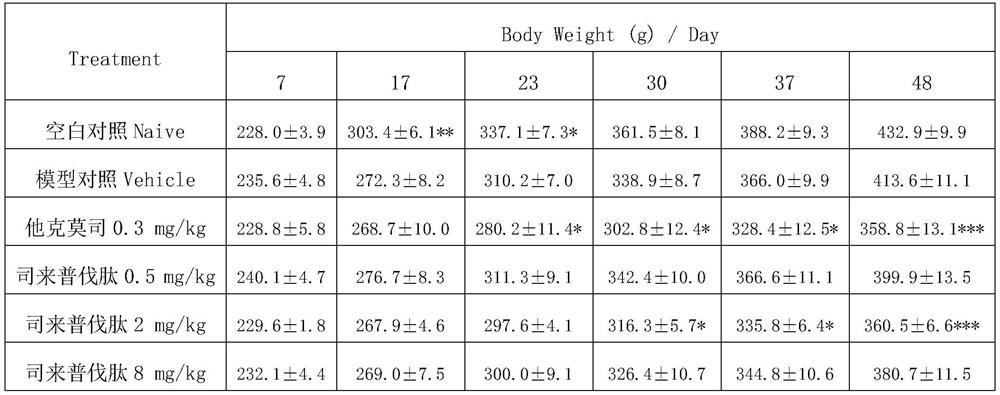
[0013] Embodiment 1: The present invention discovers the new application of selepravatide, the application of selepravatide in the preparation of medicines for treating nephropathy, specifically, as the application of preparation of medicines for treating membranous nephropathy, the application of selepravatide in the preparation of When preparing a drug for treating membranous nephropathy, the podocytes are stimulated to express MC1R, thereby reducing proteinuria and oxidative stress, and simultaneously reducing systolic blood pressure. The route of administration can be any one of intravenous injection, subcutaneous injection and inhalation.
[0014] Use of the present invention is described below by experiment:
[0015] 1. Basic information of selepravatide:
[0016] Generic name: selepravatide gel (CDE has confirmed the name)
[0017] English name: Cyslyprovatide Gel
[0018] Pinyin: Silaipufatai Ningjiao
[0019] Chemical name: (acetyl-cysteine-lysine-proline-valine-a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com