Application of hydrangea paniculata extract in treatment of chronic kidney disease by regulating intestinal flora
A technology to regulate intestinal flora and panicle hydrangea, applied in the field of biomedicine, can solve problems such as inconsistent treatment effects and serious side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 c-BSA (cationized-BSA, cationic albumin) induced chronic kidney disease model
[0058] 1. Antigen cationized BSA (C-BSA) preparation
[0059] Fraction V of natural bovine serum albumin: pure by electrophoresis, with an isoelectric point of 4.5, from Ameresco. Carbodiimide (EDC): Beijing Chemical Reagent Company. Anhydrous ethylenediamine (EDA): analytically pure, Beijing Chemical Reagent Company. Refer to the Border method, add 67mL EDA to 500mL double distilled water, then slowly add 350mL 6M hydrochloric acid, adjust the pH to 4.75, cool the solution to 25°C on ice, dissolve 5g BSA in 25mL double distilled water, and then slowly add this solution EDA solution was stirred continuously, 1.8 g of EDC was added, reacted at a constant temperature of 25 ° C for 2 h, and then terminated with 30 mL of acetic acid buffer solution of pH 4.75 to obtain a C-BSA solution with an increased isoelectric point. Dialyze the C-BSA solution with double-distilled water at 4°C...
Embodiment 2
[0063] The preparation of embodiment 2 hydrangea paniculata (Hydrangea paniculata Sieb) extract
[0064] Pane hydrangea, Saxifragaceae, stems were purchased from Jinxiu County, Guangxi Zhuang Autonomous Region in May 2014. Guangxi Liuzhou Forestry Bureau identified the samples. The sample specimen ID number is 4645, which currently exists in China, Institute of Materia Medica, Chinese Academy of Medical Sciences, Beijing.
[0065] Preparation of total coumarin glycosides from Hydrangea paniculata extract: Grind Hydrangea paniculata branches, add 10 times the amount of water to reflux and extract twice, 3 hours each time, filter the extract while it is hot, combine the filtrates, take the filtrate and balance it with water in advance The macroporous adsorption resin column (HPD100) was first used to elute the impurities with water 3 times the volume of the macromolecular polymer, and the water was discarded. Then use 17% ethanol with 5 times the volume of the macromolecular p...
Embodiment 3
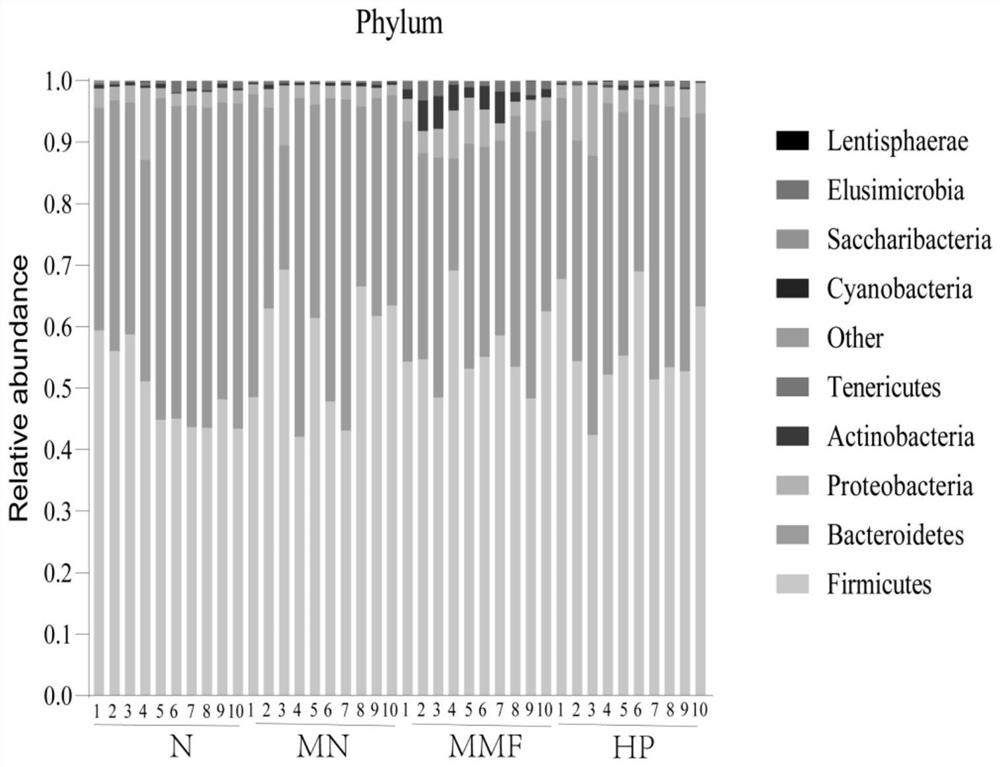
[0090] Example 3 Regulate the animal experiment of chronic kidney disease intestinal flora
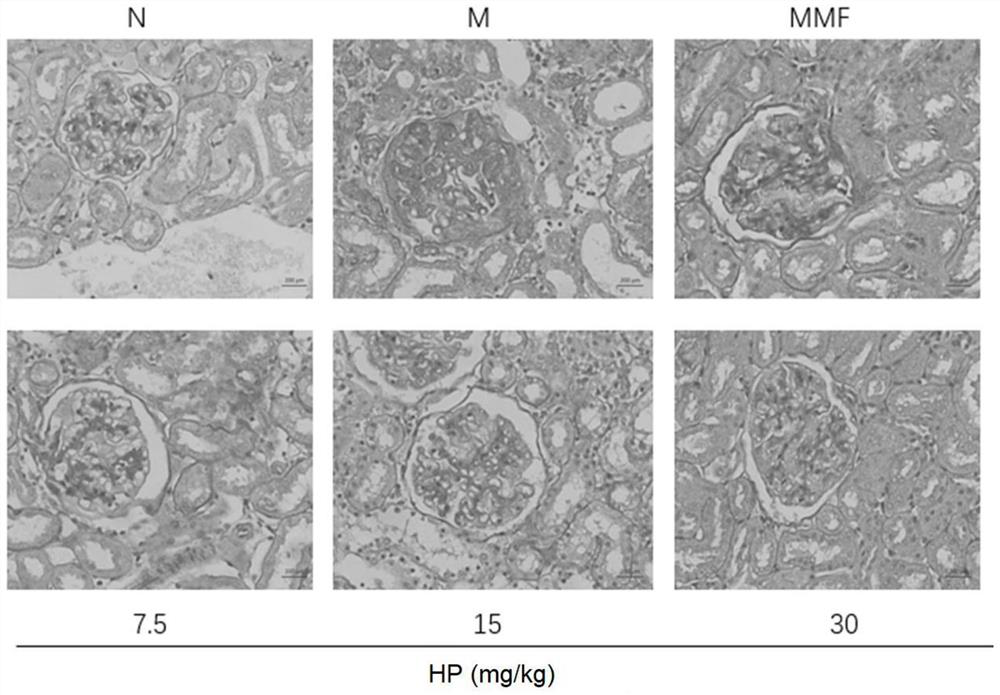
[0091] 1. 84 SD female mice (Speyford (Beijing) Biotechnology Co., Ltd.) were grouped into normal group, model (M) group, positive drug mycophenolate mofetil (MMF 20mg / kg) group, HP ( 7.5mg / kg, 15mg / kg, 30mg / kg) groups, 14 rats in each group, were administered orally once a day for 2 months in total, and various indicators were detected.
[0092] 2. Observation items:
[0093] (1) Functional examination: ① Quantitative determination of urine protein. ②Determination of serum creatinine, urine creatinine and serum urea nitrogen.
[0094] The results are shown in Table 2. Hydrangea paniculata extract can effectively reduce the body’s urinary albumin and serum creatinine, effectively improve renal function, and at the same time regulate the levels of total cholesterol, high-density lipoprotein, low-density lipoprotein and triglyceride in serum , regulate lipid metabolism.
[0095] (2) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com