Levofolinic acid freeze-dried powder injection for injection and production method thereof
A technology of levofolinic acid and freeze-dried powder injection, applied in the field of medicine, can solve the problems of complex freeze-drying process, long cycle, high energy consumption, etc., and achieve the effect of high content, reduced dosage, and reduced impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
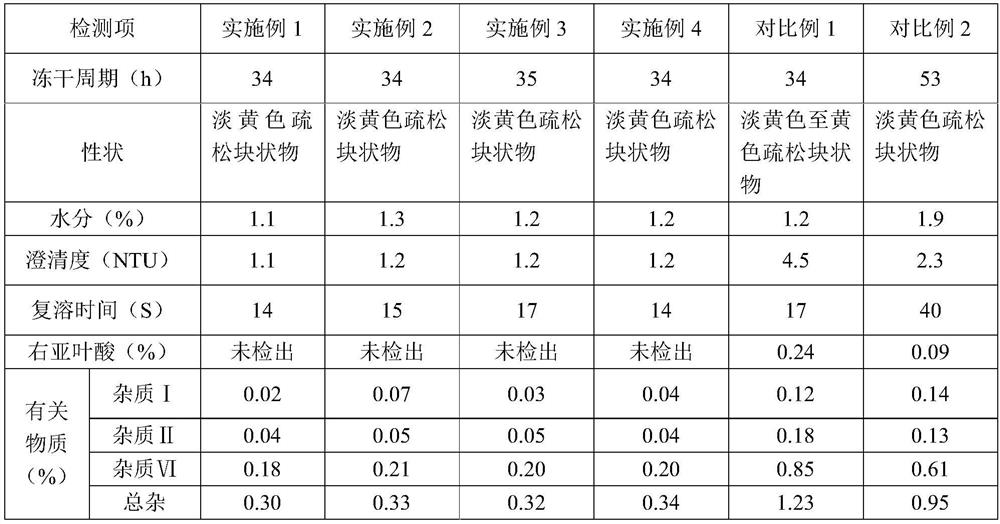
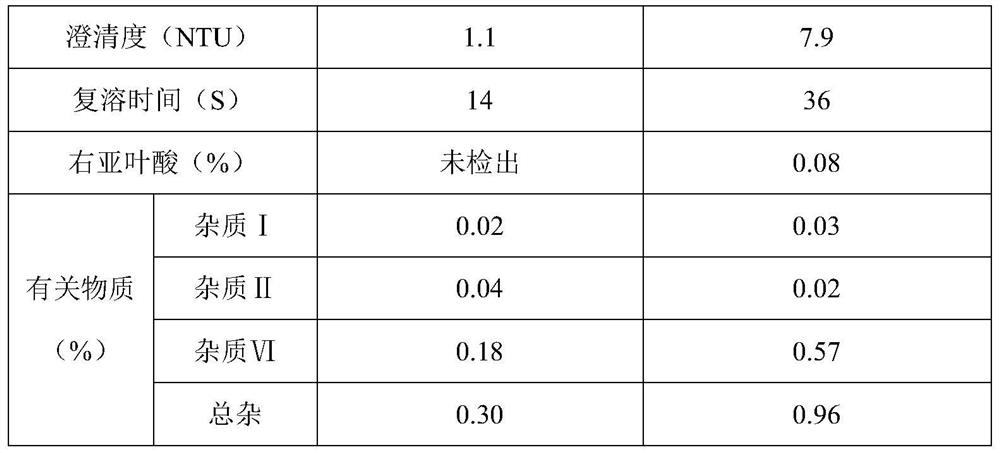
[0040] A levofolinic acid freeze-dried powder for injection, which is prepared from raw material levofolinic acid, sodium hydroxide and water for injection at a temperature of 10°C, sterilized and filtered, filled, and freeze-dried to obtain a finished product. Wherein, during the preparation process of the medicinal liquid, the pH value thereof is adjusted to 8.0 with a pH regulator, and each 1000 parts of the medicinal liquid contains the following components by weight: 50 parts of the active raw material levofolinic acid, hydrogenated Sodium 8 parts.
[0041] The detailed preparation process is as follows:
[0042] (1) Add a total of 80% of water for injection at 10°C in the liquid preparation tank, start stirring and continue to fill with nitrogen for protection, until the residual oxygen in the solution is less than 10ppm, add 50 parts of the raw material levofolinic acid and sodium hydroxide in sequence , wherein the levofolinic acid needs to be dispersed completely bef...
Embodiment 2
[0054] A levofolinic acid freeze-dried powder injection for injection, which is prepared from raw material levofolinic acid, sodium hydroxide and water for injection at a temperature of 20°C, sterilized and filtered, filled, and freeze-dried to obtain a finished product. Wherein, during the preparation process of the medicinal liquid, the pH value thereof is adjusted to 8.0 with a pH regulator, and each 1000 parts of the medicinal liquid contains the following components by weight: 50 parts of the active raw material levofolinic acid, hydrogenated Sodium 8 parts.
[0055] The detailed preparation process is as follows:
[0056] (1) Add a total of 80% of water for injection at 20°C in the liquid preparation tank, start stirring and continue to fill with nitrogen for protection, until the residual oxygen in the solution is less than 10ppm, add 50 parts of the raw material levofolinic acid and sodium hydroxide in sequence , wherein the levofolinic acid needs to be dispersed comp...
Embodiment 3
[0064] Except that freeze-drying process is different from embodiment 2, other content is identical with embodiment 2, and the process of freeze-drying is specifically as follows:
[0065] (1) The filled liquid medicine is fed at 15°C;
[0066] (2) Continue to cool down to -40°C and keep for 3 hours;
[0067] (3) Control the vacuum degree to 0.01Mpa~0.05Mpa, raise the temperature to -5°C, the heating time is 3 hours, and the holding time is 8 hours;
[0068] (4) Control the vacuum degree to 0.01Mpa-0.05Mpa, raise the temperature to 30°C, the heating time is 3 hours, and the holding time is 18 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com