Chromene-pyridine derivative fluorescent probe, preparation method and application
A technology of fluorescent probes and derivatives, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve problems such as the inability to realize hypochlorite, pH working range research, and limit practical applications, so as to improve selectivity and sensitivity, easy availability of raw materials, and wide application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation method of the chromene-pyridine derivative fluorescent probe of this embodiment is as follows:
[0032] 2.102 g of 3H-benzo[f]chromene-2-carbaldehyde (10 mmol) and 2.87 g of 4-methyl-1-(2-morpholin-4-yl-ethyl)-pyridine bromide (10 mmol ) was dissolved in 0.05L ethanol, 0.017 g piperidine (0.2 mmol) was added dropwise as a catalyst, refluxed and stirred at 80°C for 4-5h, cooled and left to room temperature, filtered under reduced pressure, the obtained solid was washed with ethanol, and then ethanol The chromene-pyridine derivative fluorescent probe was obtained by recrystallization. The yield of the target product was 84%.
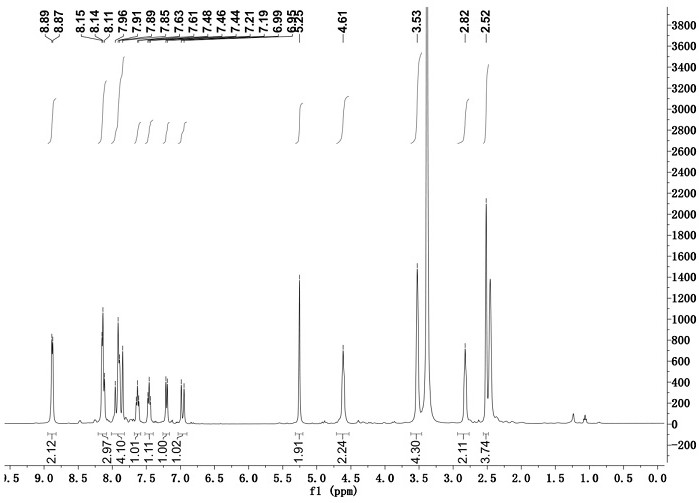
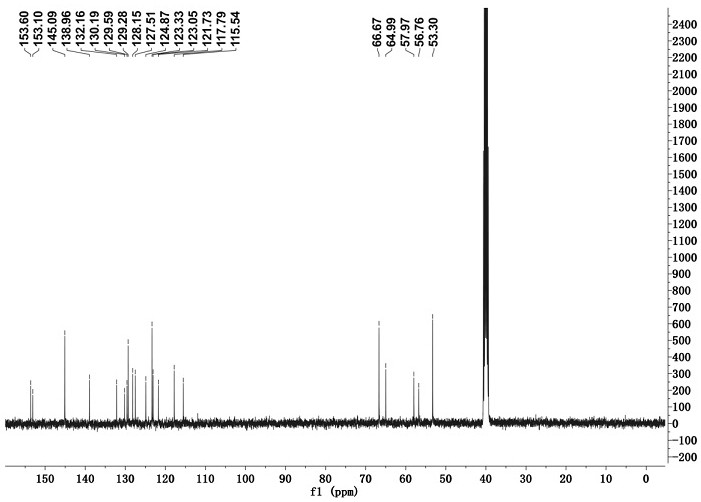
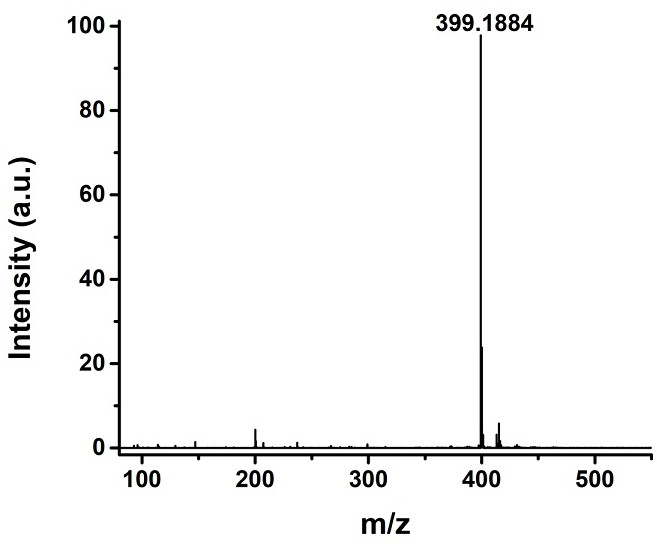
[0033] Adopt nuclear magnetic resonance instrument to carry out nuclear magnetic resonance analysis to the chromene-pyridine derivative that makes, the result is as follows:
[0034] 1 H NMR (400 MHz, DMSO- d 6), δ (ppm): 8.87-8.89 (2H, d, Ar-H), 8.11-8.15(3H, m, Ar-H), 7.85-7.96 (4H, m, Ar-H), 7.61-7.63 ( 1H, t, Ar-H), 7.44-7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com