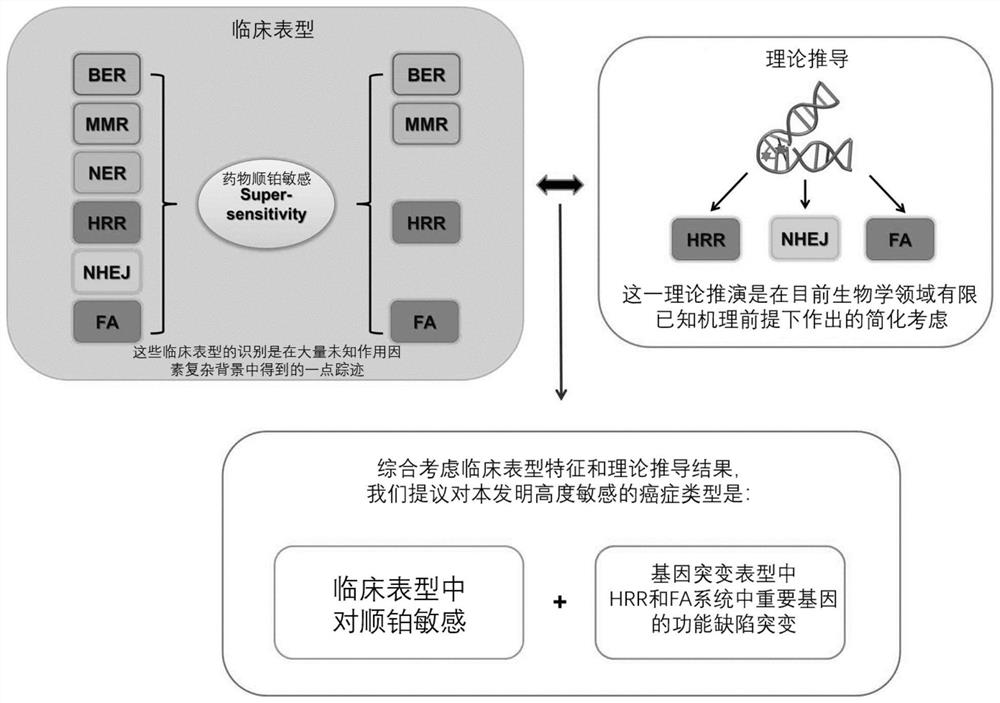
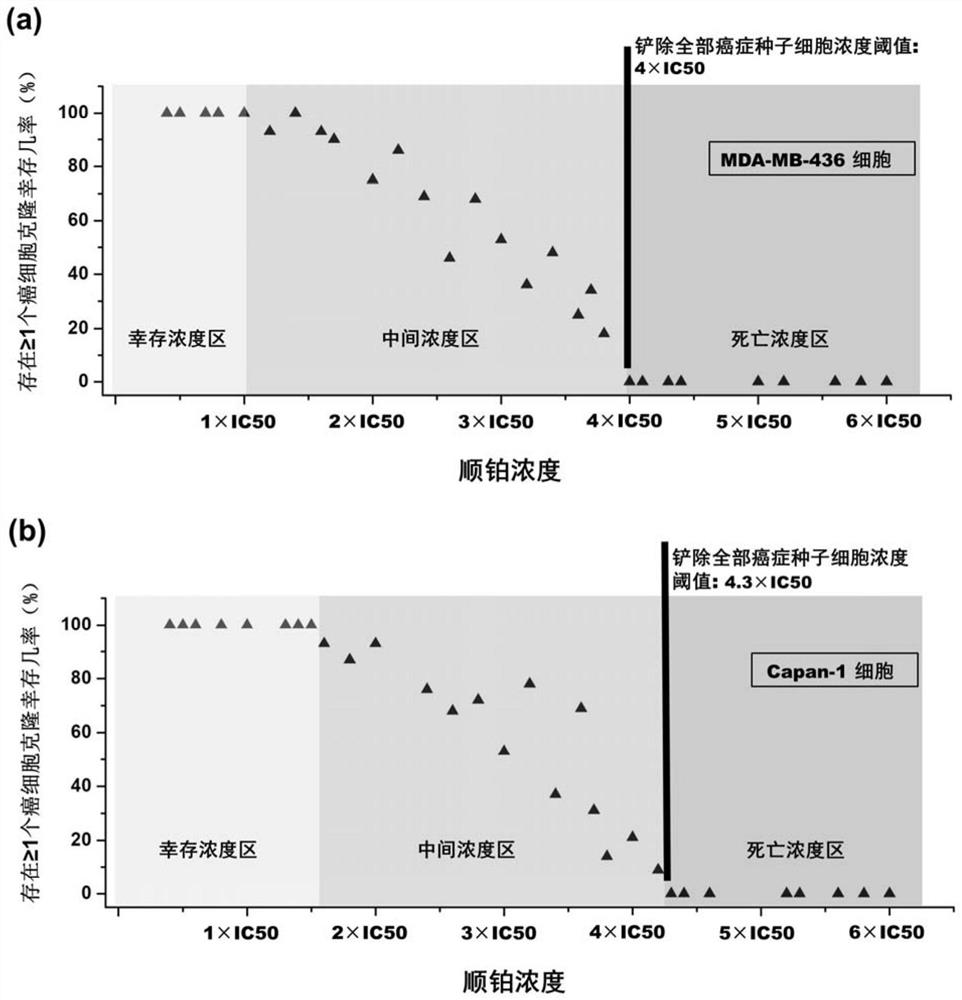
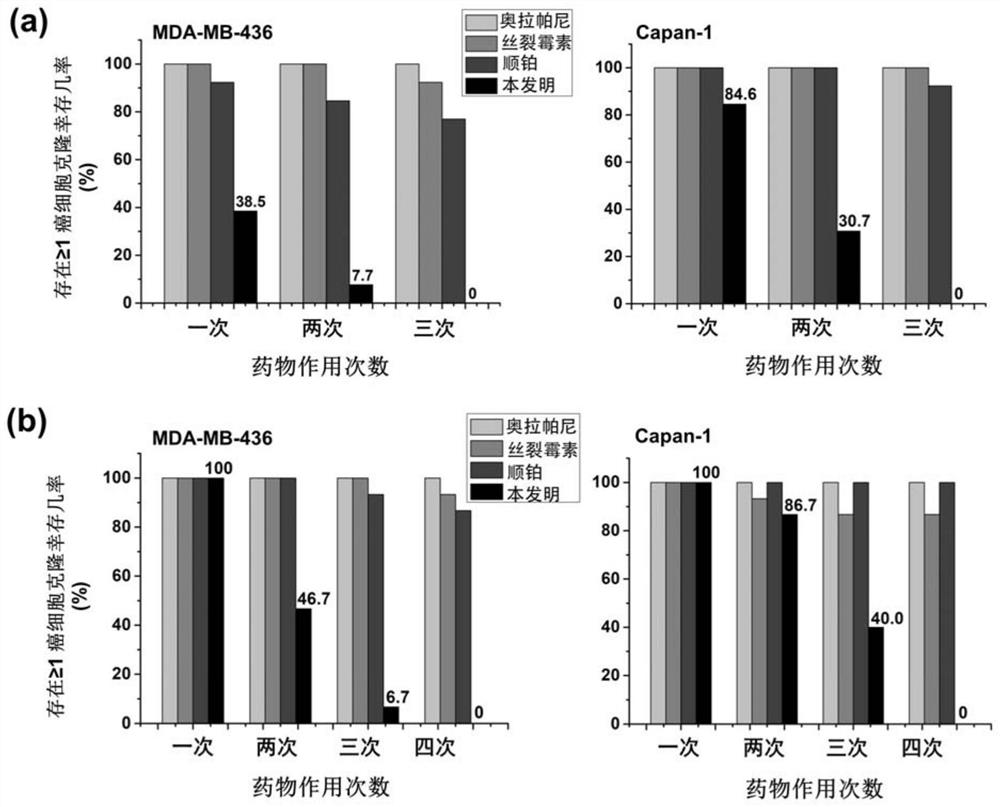
Anti-cancer composition, combination product and preparation method and application of anti-cancer composition
A combined product and composition technology, applied in the field of medicine, can solve the problems of not being able to significantly improve the survival rate of patients with cancer indications, drug failure, drug resistance, etc., and achieve the effect of low cancer recurrence rate and convenient prevention and treatment of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] This example is used to illustrate the treatment results of the method of the present invention on different cancer cells.
[0055] (1) Drug dissolution: cisplatin and transplatinum powders are directly dissolved in sterile saline, and the concentration of cisplatin can easily reach 0.3-0.6mg / ml; Shake continuously in a water bath at ℃ for 30 min. The dissolved drug solution is filtered through a 0.22 μm sterile filter and used fresh or stored at -20 ℃ for use within one week.
[0056] (2) Drug treatment plan for cancer cells:
[0057] Simulate multiple dosing regimens in the clinical chemotherapy program (that is, use 3-4 times, and the interval between each dose is 3 to 4 weeks according to the growth of cancer cells. For cancer cells, select MDA-MB-436 breast cancer cells and Capan -1 Pancreatic cancer cells, the culture method is that the first three generations of cells after recovery completely follow the culture and passage methods of the commercial instructions...
Embodiment 2
[0065] This example is used to illustrate the safety experiment of the method of the present invention.
[0066] Through human normal primary cells and rat drug safety experiments, the drug concentration of the method of the present invention is within the low dose range (2-3μM) in clinical application, and does not cause any observable effects on human primary normal cells and rats. toxicity.
[0067] Specific steps: Human primary normal cells were purchased from the National Experimental Cell Resource Sharing Platform (Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, Beijing) and ATCC (purchased by Beijing Zhongyuan Heju Economic and Trade Co., Ltd. in the United States), and cultured according to the method of purchasing the product manual , used within 6-8 passages. The drug treatment method is the same as the aforementioned "Cancer Drug Treatment Scheme". The concentration of the drug was selected in a series of doses that could cause cytotoxici...
Embodiment 3
[0072] This example is used to illustrate the effectiveness experiment of the method of the present invention - to overcome the formation of drug resistance.
[0073] Suitable for sensitive cancer cells As mentioned above, ~10,000 cancer cells (thawed in the same batch, low-passage cells p3-p5) were inoculated into 25cm 2 In the cell culture bottle, incubate and adhere to the wall in a CO2 incubator at 37°C for 2 hours, add the corresponding drug solution to the bottle to make the final concentration ~2×IC50, and then put the drug in the incubator for 2 hours (cisplatin, silk split mycin and the present invention, the method is as described above) or 7 days (olaparib, the method is as described above), and then washed with PBS, and then added fresh medium. After culturing for 3-5 weeks according to the cell growth rate, subculture the cells, and inoculate ~10,000 cancer cells / 25cm 2 Cell culture flasks, after treating the cells with gradually increasing drug concentration, ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com