Synthesis method of 7-amino-5-bromoquinoline
A synthesis method and technology of bromoquinoline, applied in directions such as organic chemistry, can solve the problem of not synthesizing 7-amino-5-bromoquinoline and the like, and achieve the effects of simple synthesis route, low cost of raw materials, and reasonable process selection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The present invention will be further described below in conjunction with specific examples, but the examples are only exemplary and do not constitute any limitation to the scope of the present invention. Those skilled in the art should understand that the details and forms of the technical solutions of the present invention can be modified or replaced without departing from the spirit and scope of the present invention, but these modifications and replacements all fall within the protection scope of the present invention.
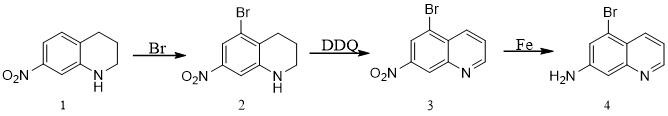
[0020] The invention provides a synthesis process of 7-amino-5-bromoquinoline, which uses 7-nitro-1,2,3,4-tetrahydroquinoline as raw material to obtain 5-bromo-7-quinoline through bromination reaction. Nitro 1,2,3,4-tetrahydroquinoline, react with DDQ dehydrogenation to obtain 5-bromo-7-nitroquinoline, obtain 7-amino-5-bromoquinoline through reduction reaction of iron powder, the reaction style such as figure 1 As shown, the specific synthesis step...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com