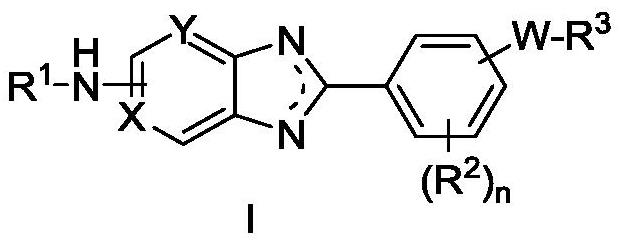
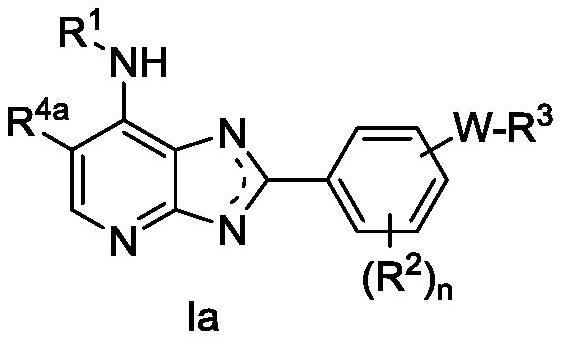
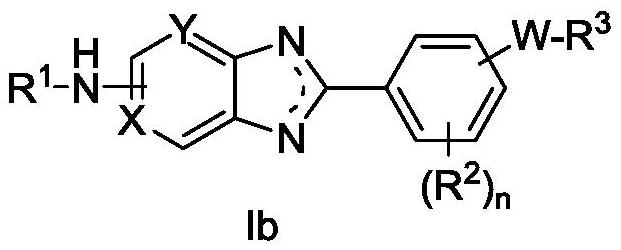
Heteroaryl compound and application thereof
A technology of heteroaryl compounds, applied in the field of heteroaryl compounds, can solve the problems of insufficient research on inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0409] Compound 1: 2-(4-(6-chloro-7-((1-((6-methoxypyridin-3-yl)methylene)piperidin-4-yl)amine)-3hydro-imidazole [4,5-b]pyridin-2-yl)phenoxy)-N-methylacetamide
[0410]
[0411] General Method E: Related Compound 5-Chloro-N 4 -(1-((6-methoxypyridin-3-yl)methylene)piperidin-4-yl)-3-nitropyridine-2,4-diamine (I-27, 50mg, 1eq) and 2-(4-formylphenoxy)-N-methylacetamide (I-1, 25 mg, 1 eq).
[0412] 1 H NMR (400MHz, DMSO-d 6 )δ13.15(s,1H),8.17–8.02(m,4H),7.92(s,1H),7.66(dd,J=8.5,2.4Hz,1H),7.17–7.07(m,2H),6.81 (d,J=8.4Hz,1H),5.81(d,J=8.8Hz,1H),5.04–4.85(m,1H),4.57(s,2H),3.84(s,3H),3.47(s, 2H), 2.86(d, J=11.1Hz, 2H), 2.68(d, J=4.6Hz, 3H), 2.15(t, J=11.5Hz, 2H), 1.98(d, J=8.7Hz, 3H) ,1.67(q,J=10.7,9.9Hz,2H).MS:ESI(+)[M+1] + 536.0.
Embodiment 2
[0414] Compound 2: 2-(4-(6-chloro-7-((1-((5-methoxypyridin-2-yl)methylene)piperidin-4-yl)ammonia)-3hydro-imidazole [4,5-b]pyridin-2-yl)phenoxy)-N-methylacetamide
[0415]
[0416] General Method E: Related Compound 5-Chloro-N 4 -(1-((5-methoxypyridin-2-yl)methylene)piperidin-4-yl)-3-nitropyridine-2,4-diamine (I-28,1eq) and 2 -(4-Formylphenoxy)-N-methylacetamide (I-1, 1 eq).
[0417] 1 H NMR (400MHz, DMSO-d 6)δ13.15(s,1H),8.24(s,1H),8.08(d,J=8.6Hz,3H),7.92(s,1H),7.48–7.31(m,2H),7.21–7.06(m ,2H),5.84(d,J=8.6Hz,1H),4.98(s,1H),4.57(s,2H),3.83(s,3H),3.65(s,2H),2.94(s,2H) ,2.68(d,J=4.6Hz,3H),2.31(d,J=20.6Hz,2H),2.02(d,J=11.6Hz,2H),1.72(d,J=12.2Hz,2H); MS :ESI(+)[M+1] + 536.0.
Embodiment 3
[0419] Compound 3: 2-(4-(6-chloro-7-((1-(4-(trimethylsilyl)benzyl)piperidin-4-yl)amino)-3hydro-imidazol[4,5 -b]pyridin-2-yl)phenoxy)-N-methylacetamide
[0420]
[0421] General Method E: Related Compound 5-Chloro-3-nitro-N 4 -(1-(4-(trimethylsilyl)benzyl)piperidin-4-yl)pyridine-2,4-diamine (I-29,1eq) and 2-(4-formylphenoxy )-N-methylacetamide (I-1, 1 eq).
[0422] 1 H NMR (400MHz, DMSO-d 6 )δ7.95–7.77(m,3H),7.67(s,1H),7.25(d,J=7.5Hz,2H),7.09(d,J=7.6Hz,2H),6.95–6.80(m,2H ),5.53(d,J=8.8Hz,1H),4.73(d,J=10.9Hz,1H),4.32(s,2H),3.27(s,3H),2.63(d,J=11.0Hz,2H ), 2.44(d, J=4.6Hz, 3H), 1.91(t, J=11.4Hz, 2H), 1.75(d, J=11.3Hz, 2H), 1.44(td, J=12.8, 9.3Hz, 2H ); MS:ESI(+)[M+1] + 576.9.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com