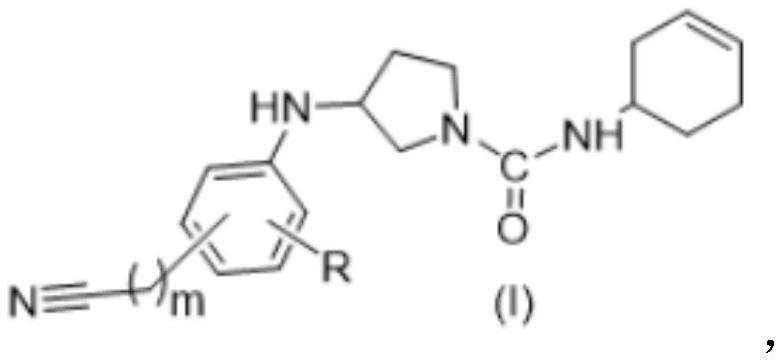
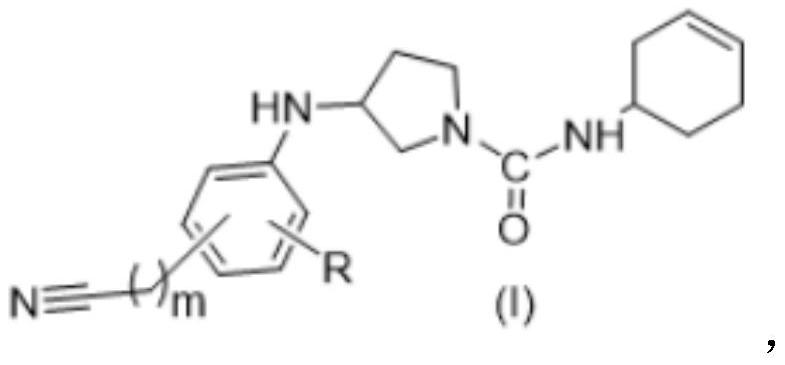
Nitrile substituted phenyl compound and application thereof
A compound, aminoacyl technology, applied in the field of nitrile-substituted phenyl compounds, can solve the problems of side effects, peripheral neurotoxicity, toxicity, etc., and achieve the effect of high safety and good tumor inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation of embodiment 1 compound 1
[0046]
[0047] Step 1 Preparation of compound 1a
[0048]
[0049] In a 500mL single-necked bottle, add 1,4-dioxane (200mL), then sequentially add 3-cyclohexene-1-amine hydrochloride (11g), triphosgene (8.86g) and triethylamine ( 4.02g), reacted at 130°C for 2h. TLC showed that the reaction was complete, and the solvent was removed by rotary evaporation to obtain a crude product, which was separated and purified by column chromatography to obtain the title compound. ESI-MS m / z:[M+H] + =124.3.
[0050] Step 2 Preparation of compound 1b
[0051]
[0052] Dissolve 3-chloropyrrolidine (4g) in 25mL N,N-dimethylformamide, add sodium hydride (2g) under stirring at -5°C, continue stirring for 0.5h, then add compound 1a (9g), and raise room temperature After stirring for 1 h, the reaction was monitored by LC-MS, and the reaction solution was directly injected into the next step. ESI-MS m / z:[M+H] + =229.1.
[0053] St...
Embodiment 2
[0062] The preparation of embodiment 2 compound 2
[0063]
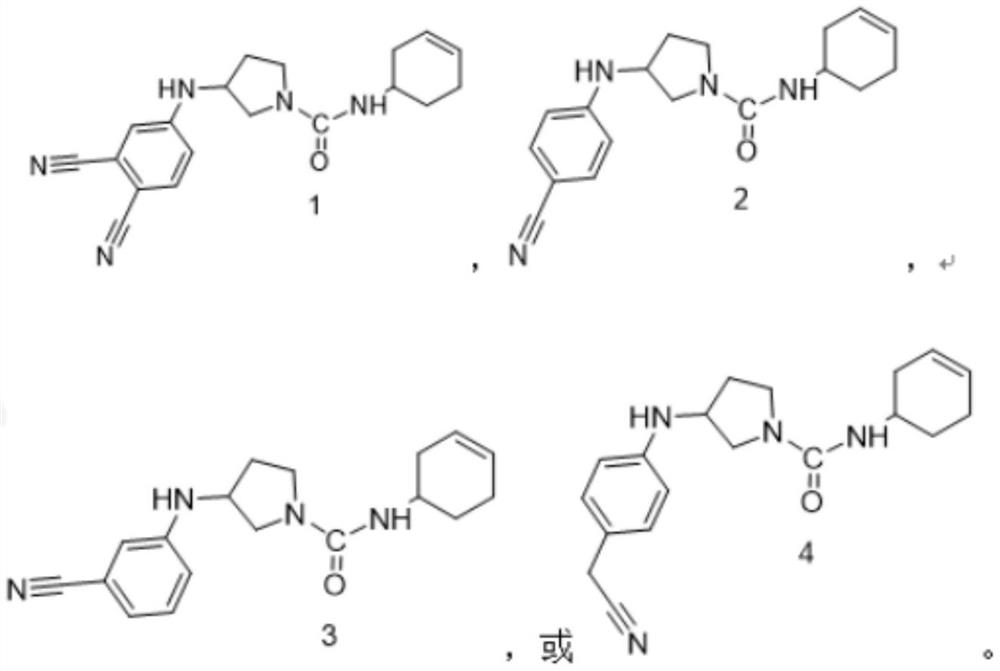
[0064] The preparation method was the same as that in Example 1, except that 4-aminophthalonitrile was replaced by p-aminobenzonitrile to obtain the title compound. ESI-MS m / z:[M+H] + = 311.1.
Embodiment 3
[0065] The preparation of embodiment 3 compound 3
[0066]
[0067] The preparation method was the same as that in Example 1, except that 4-aminophthalonitrile was replaced by m-aminobenzonitrile to obtain the title compound. ESI-MS m / z:[M+H] + = 311.1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com