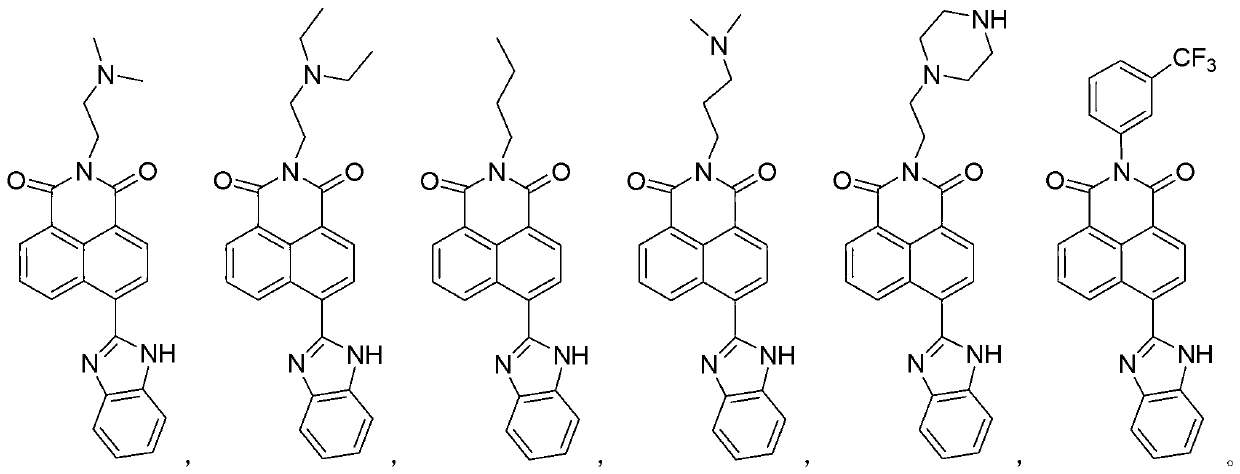
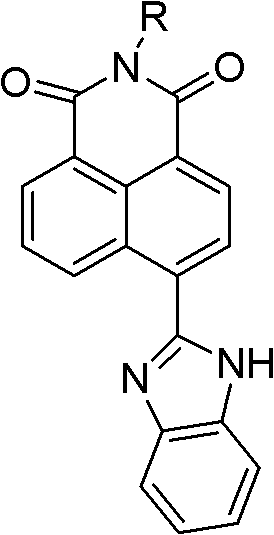
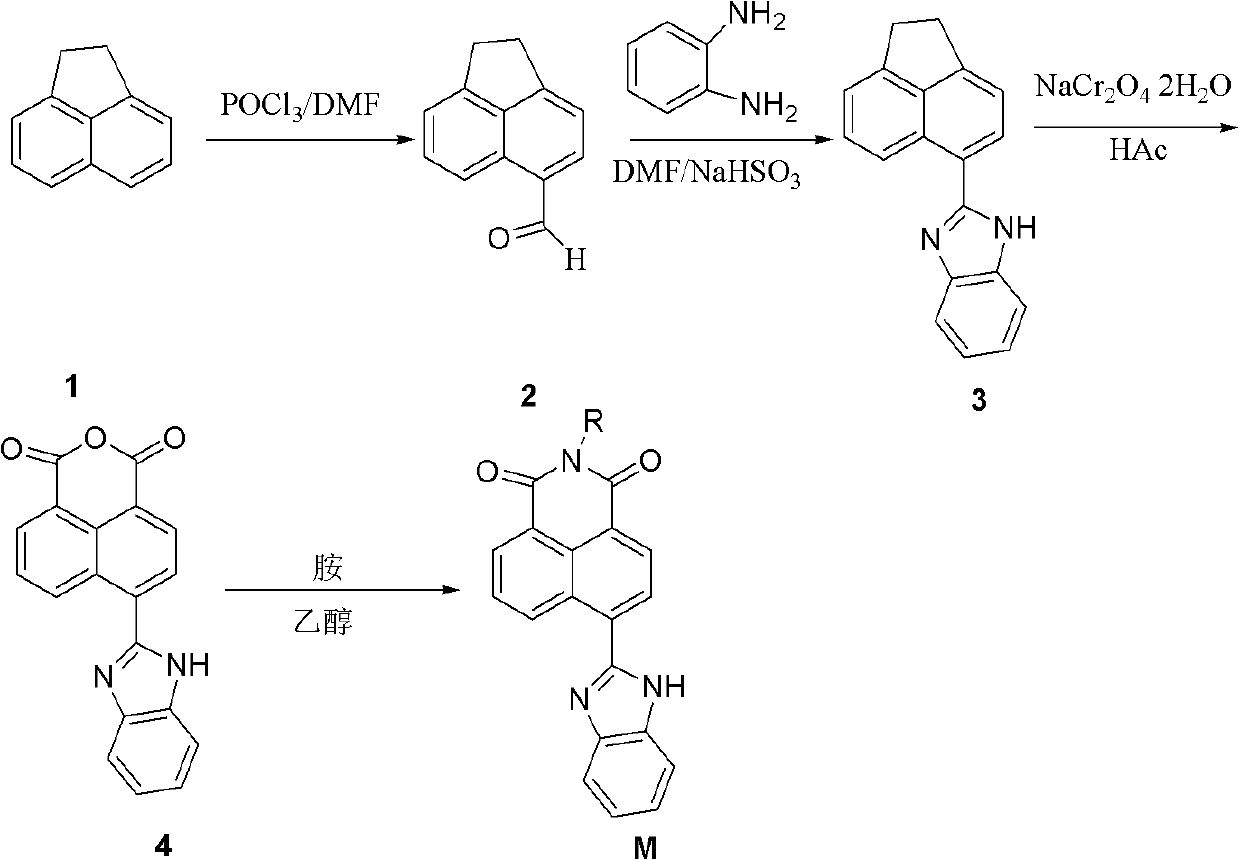
Synthesis of benzimidazole-containing naphthalimide derivatives and applications of benzimidazole-containing naphthalimide derivatives on cancer resistance
A technology of benzimidazole and naphthalimide, applied to medical preparations containing active ingredients, antineoplastic drugs, drug combinations, etc., to achieve good tumor inhibitory activity and cell selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Synthesis of N-butyl-4-(1H-benzo[d]imidazole-2-y1)-1,8-naphthalimide (derivative 3)
[0018] (1) Synthesis of 1,2-dihydroacenaphthylene-5-carbonaldehyde (2)
[0019]
[0020] In a zero-degree ice-water bath, add 10 mL (1.3 mol) of anhydrous DMF (treated with calcium hydride) to a 100 mL dry two-necked bottle, and slowly add 10 mL (1.0 mol) of DMF (treated with calcium hydride) to the reaction system with a constant pressure dropping funnel under magnetic stirring. POC13, remove the ice-water bath, react at room temperature for 1 hr, dissolve 4.0 g (26.0 mmol) of acenaphthene in 15 mL of anhydrous DMF, add dropwise into the reaction system, raise the temperature to 100 °C after the dropwise addition, continue the reaction for 8 hr, and trace to The response is complete. Cooled to room temperature, slowly poured into 200 mL of ice water under vigorous stirring, precipitated gray precipitate, filtered, dried to obtain 4.2 g of gray needle-like solid, yield: 88.9%. The...
Embodiment 2
[0033] N-(N',N'-dimethylaminoethyl)-4-(1H-benzo[d]imidazole-2-y1)-1,8-naphthalimide (derivative 1)
[0034]
[0035] Except using N,N-dimethylethylenediamine instead of n-butylamine, other preparation and purification methods are the same as in Example 1. Separated by silica gel column chromatography (column chromatography eluent: CH 2 Cl 2 :CH 3 OH=20:1) to obtain compound M1 as a yellow solid, yield: 88.9%. Melting point: 93.2-93.8°C.
[0036] HR-MS (m / z): C 23 h 20 N 4 o 2 , calculated value: 384.1586, measured value: 384.1588.
[0037] 1 HNMR(d 6 -DMSO, 400MHz): δ(ppm): 13.35(s, 1H), 8.76(d, J=8.8Hz, 1H), 8.63(d, J=7.6Hz, 1H), 8.58(d, J=6.8Hz , 1H), 8.37(d, J=7.6Hz, 1H), 8.00(dd, J 1 =6.0Hz,J 2 =5.6Hz, 1H), 7.74(m, 2H), 7.33(m, 2H), 4.19(t, J=7.2Hz, 2H), 2.60(t, J=7.2Hz, 2H), 2.27(s, 3H ).
Embodiment 3
[0039] N-(N'N'-diethylaminoethyl)-4-(1H-benzo[d]imidazole-2-y1)-1,8-naphthalimide (derivative 2)
[0040]
[0041] Except using N, N-diethylethylenediamine instead of n-butylamine, other synthesis and purification methods are the same as example 1, separated through silica gel column (column chromatography eluent is CH 2 Cl 2 :CH 3 OH=20:1) to obtain the target compound M2 as a yellow-green solid with a yield of 81.7% and a melting point of 149.9-150.6°C.
[0042] HR-MS (m / z): C 25 h 24 N 4 o 2 , calculated value: 412.1899, measured value: 412.1895.
[0043] 1 HNMR(d 6 -DMSO, 400MHz): δ(ppm): 13.22(s, 1H), 9.75(d, J=8.0Hz, 1H), 8.63(d, J=8.0Hz, 1H), 8.58(d, J=8.0Hz , 1H), 8.37(d, J=7.6Hz, 1H), 7.99(dd, J 1 =J 2 =8.0Hz, 1H), 7.84(d, J=7.6Hz, 1H), 7.66(d, J=7.6Hz, 1H), 7.32(m, 2H), 4.16(t, J=8.0Hz, 2H), 2.73(t, J=8.0Hz, 2H), 2.60(m, 4H), 0.99(s, 6H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com