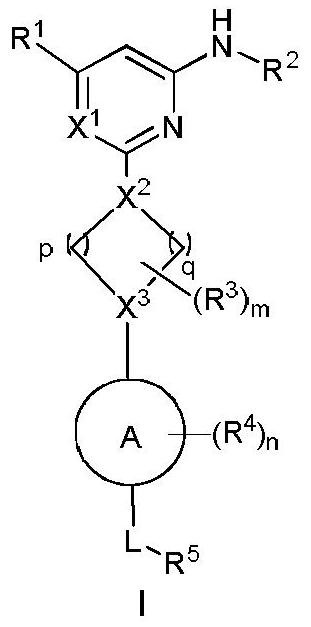
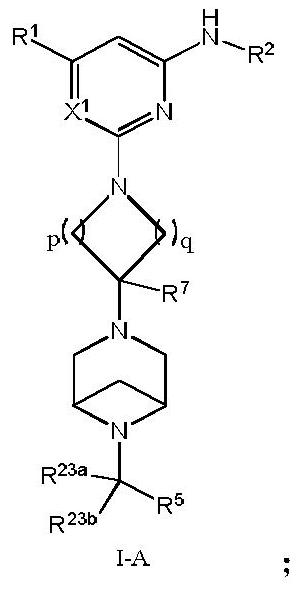
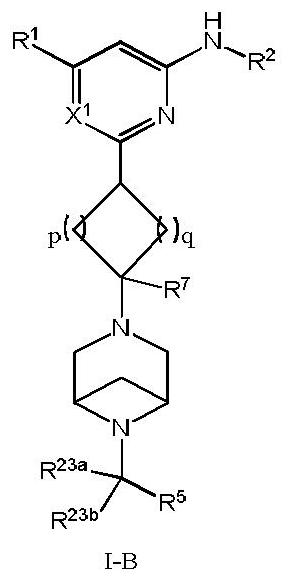
Arylamine compound, pharmaceutical composition containing arylamine compound as well as preparation method and application of arylamine compound
A compound and mixture technology, applied in the field of new arylamine compounds, can solve the problems of inhibitors on the market and achieve good inhibitory effect and good pharmacokinetic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0263] Example 1: 2-(4-(6-((6-(4-fluoro-1H-pyrazol-1-yl)pyridin-3-yl)methyl)-3,6-diazabicyclo[3.1 .1] Heptane-3-yl)piperidin-1-yl)-6-methyl-N-(5-methyl-1H-pyrazol-3-yl)pyrimidin-4-amine (Compound 1)
[0264]
[0265] The first step: 3-(1-((benzyloxy)carbonyl)piperidin-4-yl)-3,6-diazabicyclo[3.1.1]heptane-6-carboxylic acid tert-butyl ester (1b )
[0266] 4-Oxopiperidine-1-carboxylate benzyl ester (353mg, 1.5mmol), 1a (200mg, 1.0mmol) and NaBH 3 CN (317mg, 5.0mmol) was placed in a reaction flask, added to methanol (5mL) and AcOH (0.2mL) in sequence, and stirred at room temperature until the raw material was completely converted. After the reaction was completed, saturated ammonium chloride solution (0.1 mL) was added to the reaction system to quench the reaction, the solvent was concentrated under reduced pressure, and purified by Flash column chromatography (PE:EA=1:1) to obtain compound 1b (289 mg). MS (ESI, m / z): 416.3 [M+H] + .
[0267] The second step: tert-butyl 3-...
Embodiment 2
[0278] Example 2: 2-(4-(6-(1-(6-(4-fluoro-1H-pyrazol-1-yl)pyridin-3-yl)ethyl)-3,6-diazabicyclo [3.1.1] Heptane-3-yl)piperidin-1-yl)-6-methyl-N-(5-methyl-1H-pyrazol-3-yl)pyrimidin-4-amine (compound 2 )
[0279]
[0280] Compound 1f (30mg, 81μmol), 2a (25mg, 122μmol) and Ti(O i Pr) 4 (92mg, 326μmol) was placed in a reaction flask, THF (2mL) was added, heated to 75°C for 16h under nitrogen protection, and then NaBH(OAc) was added 3 (86mg, 407μmol), the reaction was continued at 75°C until the starting material was completely converted. After the reaction was completed, the reactant was cooled to room temperature, and saturated ammonium chloride solution (0.1 mL) was added to quench the reaction, the reaction solution was concentrated, and the residue was purified by Prep-TLC (DCM:MeOH=10:1) and Prep-HPLC successively. Compound 2 (3 mg) was isolated.
[0281] MS (ESI, m / z): 557.9 [M+H] + .
[0282] 1 H NMR (400MHz, DMSO-d 6 )δ11.85(s, 1H), 9.20(s, 1H), 8.65(d, J=4.5Hz,...
Embodiment 3
[0283] Example 3: 6-methyl-2-(4-(6-((6-(5-methyl-1H-pyrazol-1-yl)pyridin-3-yl)methyl)-3,6- Diazabicyclo[3.1.1]heptane-3-yl)piperidin-1-yl)-N-(5-methyl-1H-pyrazol-3-yl)pyrimidin-4-amine (Compound 3)
[0284]
[0285] Compound 1f (20mg, 54μmol), 3a (12mg, 65μmol) and Ti(O i pr) 4 (62mg, 217μmol) was placed in a reaction flask, THF (3mL) was added, heated to 75°C under nitrogen protection and stirred for 8h, then NaBH(OAc) was added 3 (58 mg, 271 μmol), the reaction was continued at 75°C until all the starting materials were converted. After the reaction, the reactant was cooled to room temperature, and saturated ammonium chloride solution (0.1 mL) was added to quench the reaction, the reaction solution was concentrated, and the residue was purified by Prep-TLC (DCM:MeOH=10:1) and Prep-HPLC successively. Compound 3 (2 mg) was isolated.
[0286] MS m / z(ESI): 539.9[M+H] + .
[0287] 1 H NMR (400MHz, DMSO-d 6 )δ11.85(s, 1H), 9.18(s, 1H), 8.45(d, J=2.4Hz, 1H), 8.35(d, J=1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com