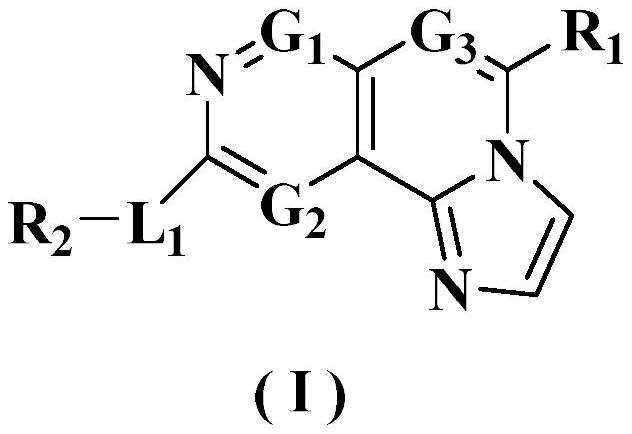
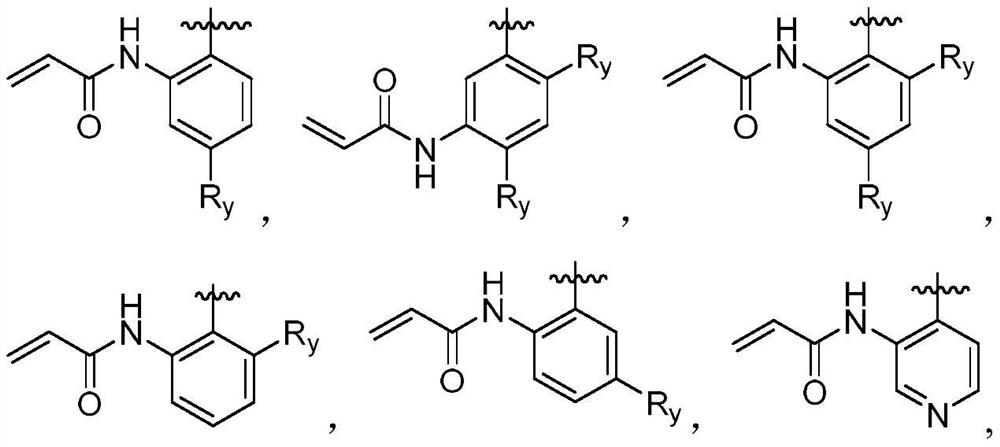
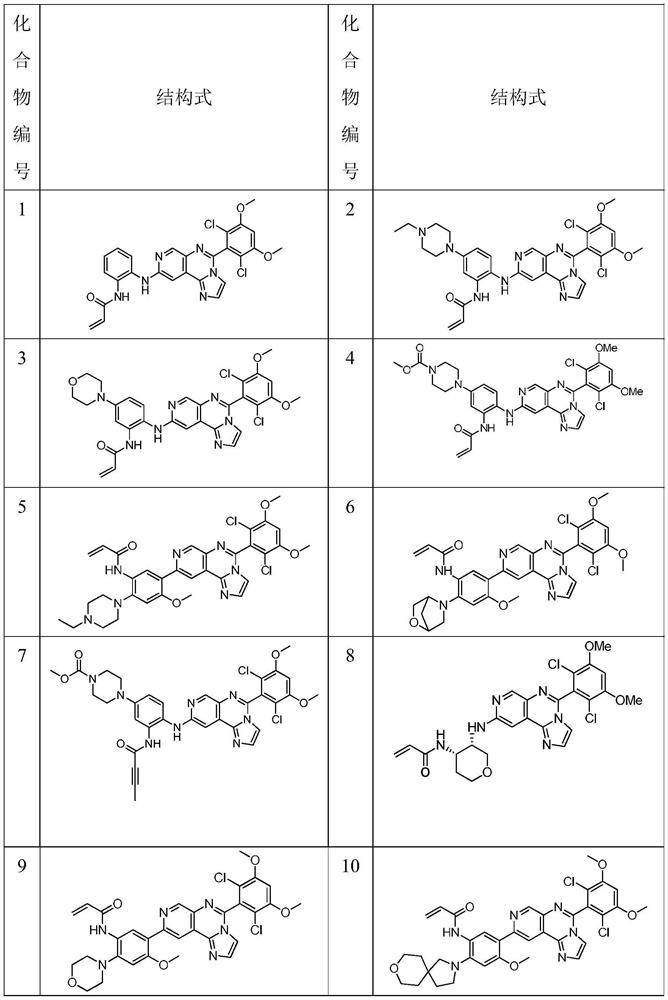
Fused tricyclic derivative as FGFR4 inhibitor
A technology of derivatives and solvates, applied in the field of fused tricyclic derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Compound 1A:
[0122]
[0123] 5-Amino-2-chloropyridine-4-carboxylic acid (24.5 g, 142 mmol) was dissolved in thionyl chloride (600 mL) at room temperature. The reaction solution was heated to 80° C. and stirred for 4 hours.
[0124] After LCMS monitoring showed that the starting material disappeared, the reaction system was cooled to room temperature. The mixture was concentrated under reduced pressure. A solution of the obtained residue in tetrahydrofuran (100 mL) was added dropwise to aqueous ammonia (600 mL) under an ice-water bath. The reaction system was slowly warmed to room temperature and stirred for 2 hours.
[0125] After LCMS monitoring showed that the starting material disappeared, water (500 mL) was added to the above reaction system to quench it. The mixture was extracted with ethyl acetate (1.0 L x 3 times), the organic phases were combined, and the organic phase was washed with saturated brine (300 ml x 3 times), then dried with anhydrous sodium ...
Embodiment 2
[0174] Compound 2A:
[0175]
[0176] tert-Butyl (4-bromo-2-nitrophenyl)carbamate (500 mg, 1.58 mmol) was dissolved in toluene (15.0 mL) at room temperature under nitrogen. Subsequently, N-ethylpiperazine (270 mg, 2.37 mmol), tris(dibenzylideneacetone) dipalladium (290 mg, 0.32 mmol), 4,5-bis-diphenyl Phosphine-9,9-dimethylxanthene (370 mg, 0.64 mmol) and anhydrous cesium carbonate (1.04 g, 3.20 mmol). The reaction solution was heated to 105°C and stirred for 3 hours.
[0177] After LCMS monitoring showed that the starting material disappeared, the reaction solution was cooled to room temperature. Water (50 mL) was added to the reaction system to quench it. The mixture was extracted with ethyl acetate (20 ml x 3 times), the organic phases were combined, the organic phase was washed with saturated brine (10 ml x 3 times), then dried over anhydrous sodium sulfate, filtered, and the obtained filtrate was decompressed Concentrate. The resulting residue was purified by sili...
Embodiment 3
[0199] Compound 3A:
[0200]
[0201] tert-Butyl (4-bromo2-nitrophenyl)carbamate (1.00 g, 3.14 mmol) was dissolved in toluene (25.0 mL) at room temperature under nitrogen. Subsequently, morpholine (411 mg, 4.72 mmol), tris(dibenzylideneacetone) dipalladium (576 mg, 0.63 mmol), 4,5-bisdiphenylphosphine-9, 9-Dimethylxanthene (728 mg, 1.26 mmol) and anhydrous cesium carbonate (2.05 g, 6.28 mmol). The reaction system was heated to 105 degrees Celsius and stirred for 3 hours.
[0202] After LCMS monitoring showed that the raw materials disappeared, the reaction system was cooled to room temperature, and water (50 ml) was added to the reaction solution to quench it. The mixture was extracted with ethyl acetate (30 mL×3 times), and the organic phases were combined. The organic phase was first washed with saturated brine (20 mL×3 times), then dried over anhydrous sodium sulfate, filtered, and finally decompressed. concentrate. The resulting residue was purified by silica gel co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com