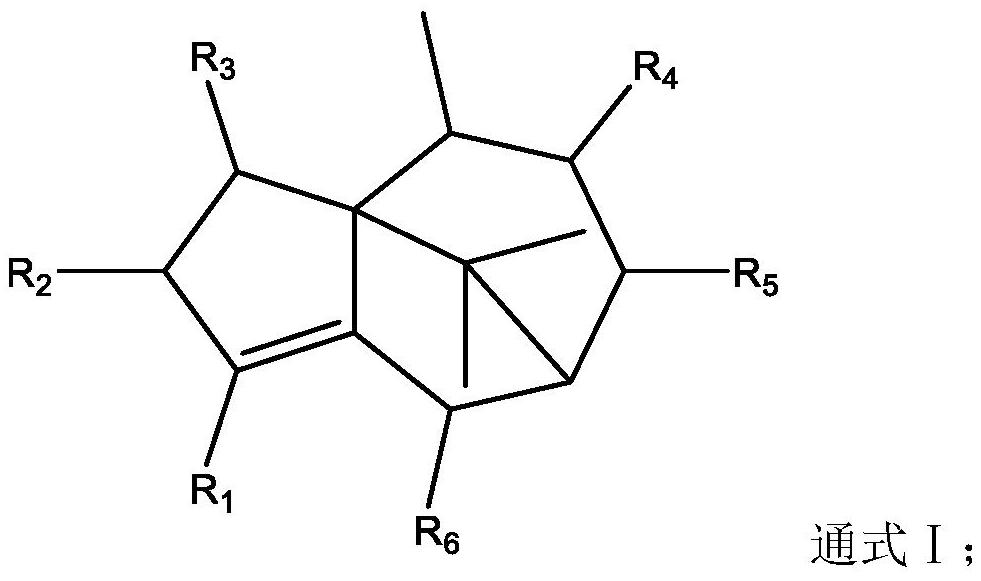
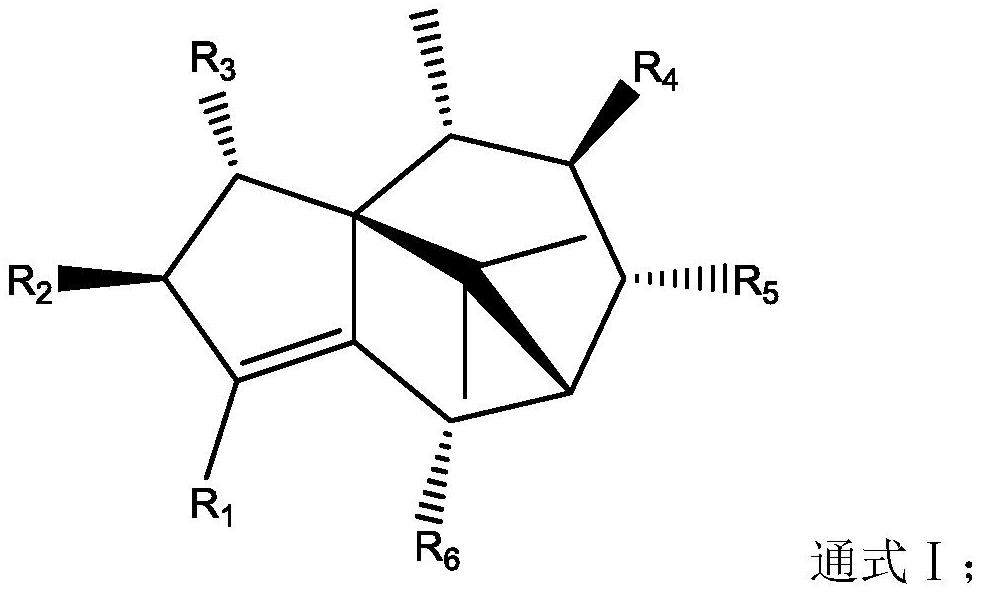
Application of terpenoids in preparation of anti-influenza virus drugs
A terpenoid, anti-influenza virus technology, applied in the direction of antiviral agents, hydroxyl compound active ingredients, anhydride/acid/halide active ingredients, etc., can solve problems such as unpredictable inhibition, and achieve excellent anti-influenza virus activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0037] Experimental example 1 in vitro anti-influenza virus activity test
[0038] Cytotoxicity test: 6×10 4 / mL MDCK cells were spread in a 96-well plate, placed in an incubator at 37°C overnight, then added a doubly diluted compound to be tested, and continued to cultivate for 48 hours, discarded the old medium, and added fresh medium containing 10% to continue to cultivate for 0.5 ~2 hours, then measure the OD value, and calculate the half lethal concentration (CC) of the compound with Graphpad Prime 6.0 software 50 ).
[0039] Plaque subtraction method: MDCK cells covered with a monolayer in a six-well plate were infected with virus (~50pfu / well), incubated in a 37°C incubator for 1 hour, and shaken every 15 minutes. The cells were washed twice with PBS, agarose containing different concentrations of compounds was added, and cultured for 48-72 hours. After the cells were fixed with paraformaldehyde for 15 minutes, they were stained with crystal violet, and then the spots...
Embodiment 2
[0056] Example 2 In vivo anti-influenza virus activity test
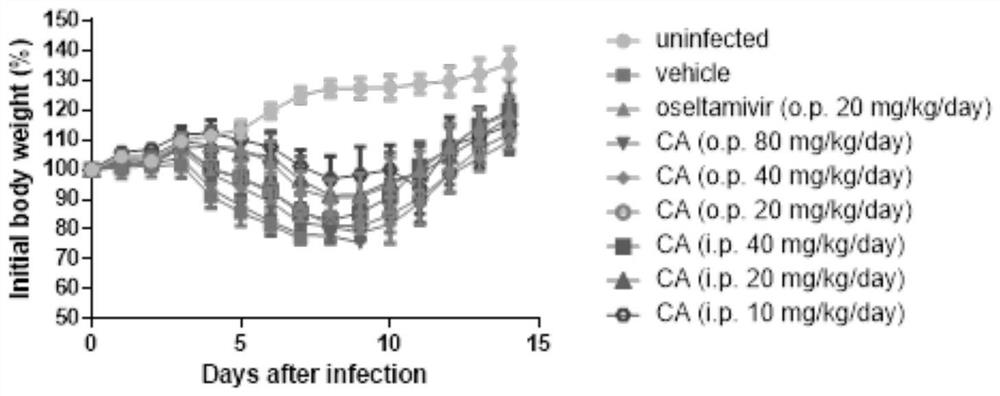
[0057] Male mice aged 4-6 weeks were randomly divided into 8 groups, 10 mice per group. Each mouse was intranasally infected with 50 μL of 2LD50 A / PR8 / 34 H1N1, and two hours later, the drugs were administered in the following groups; continuous administration for 5 days; used to test cyperenoic acid and oseltamivir phosphate (Oseltamivir) in vivo activity. After the administration, the body weight and death of the mice were recorded every day, and the records were observed for 14 days. See the experimental results figure 1 and Table 2.
[0058] Administration method and dose of CA (cyperenoic acid) test group:
[0059] (1) Oral administration, 2 times / day: high-dose group (80mg / kg / day), middle-dose group (40mg / kg / day), low-dose group (20mg / kg / day); cyperenoic acid intragastric administration The high, medium and low dose groups are abbreviated as: CA (o.p.80mg / kg / day), CA (o.p.40mg / kg / day), CA (o.p.20mg / kg / day...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com