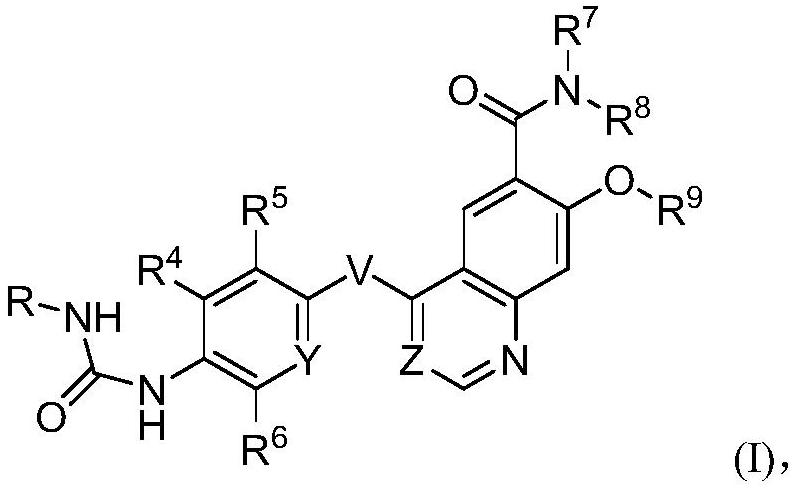
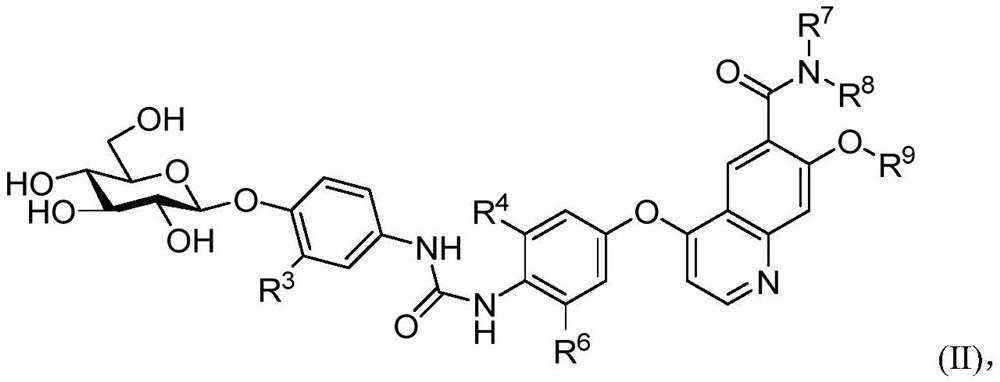
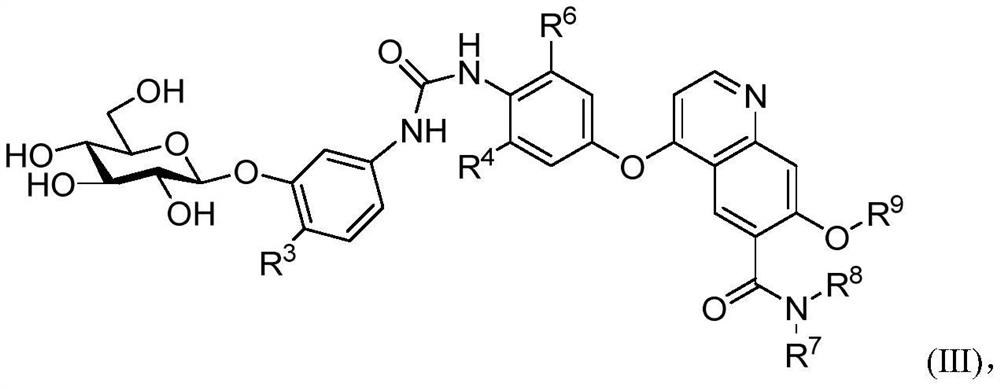
Nitrogen-containing aromatic ring derivatives containing glucose and uses thereof
A technology of medicines and compounds, applied in the fields of diseases, nitrogen-containing aromatic ring derivatives and pharmaceutical compositions, preparation of such compounds and pharmaceutical compositions, prevention or treatment of tumors, nitrogen-containing aromatic ring derivatives and pharmaceutical compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0160] Example 1 4-(3-Chloro-4-(3-(4-(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro Synthesis of -2H-pyran-2-yl)oxy)phenyl)urea)phenoxy)-7-methoxyquinoline-6-carboxamide
[0161]
[0162] Step 1) (2R,3R,4S,5R,6R)-2-(acetoxymethyl)-6-(2,2,2-trichloro-1-iminoethoxy)tetra Synthesis of Hydrogen-2H-pyran-3,4,5-triyltriacetate
[0163]
[0164] (2R,3R,4S,5R)-2-(acetoxymethyl)-6-hydroxytetrahydro-2H-pyran-3,4,5-triyltriacetate (4.0 g, 11.5 mmol) , trichloroacetonitrile (11.5mL, 115mmol), 4A molecular sieves (5g) and dichloromethane (80mL) were added to a 250mL single-neck round-bottomed flask, stirred in an ice bath under nitrogen protection for 1 hour, and then added DBU (0.35mL, 2.3mmol) ), stirred the reaction under ice bath for 1 hour; stopped the reaction, filtered, collected the filtrate and spin-dried under reduced pressure, separated and purified by column chromatography (petroleum ether / ethyl acetate (v / v)=4 / 1) to obtain the title compound as pale...
Embodiment 2
[0186]Example 2 4-(3-Chloro-4-(3-(3-Fluoro-4-((((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl) Synthesis of tetrahydro-2H-pyran-2-yl)oxy)phenyl)urea)phenoxy)-7-methoxyquinoline-6-carboxamide
[0187]
[0188] Step 1) (2R,3R,4S,5R,6S)-2-(acetoxymethyl)-6-(2-fluoro-4-nitrophenoxy)tetrahydro-2H-pyridine Synthesis of Furan-3,4,5-triyltriacetate
[0189]
[0190] The title compound in this step was prepared according to the method described in Example 1, Step 2, namely (2R,3R,4S,5R,6R)-2-(acetoxymethyl)-6-(2,2,2-trichloro) -1-Imineethoxy)tetrahydro-2H-pyran-3,4,5-triyltriacetate (1.0 g, 2.03 mmol), 2-fluoro-4-nitro-phenol (382 mg, 2.43mmol), 4A molecular sieves (1.2g) and boron trifluoride ether (0.316mL, 2.43mmol) were prepared by reacting in dichloromethane (16mL), and the crude product was separated and purified by silica gel column chromatography (petroleum ether / ethyl acetate) (v / v)=2 / 1) to give the title compound as a white solid (0.65 g, 65.7%).
[0191] MS...
Embodiment 3
[0209] Example 3 4-(3-Chloro-4-(3-(3-Chloro-4-((((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl) Synthesis of tetrahydro-2H-pyran-2-yl)oxy)phenyl)urea)phenoxy)-7-methoxyquinoline-6-carboxamide
[0210]
[0211] Step 1) (2R,3R,4S,5R,6S)-2-(acetoxymethyl)-6-(2-chloro-4-nitrophenoxy)tetrahydro-2H-pyridine Synthesis of Furan-3,4,5-triyltriacetate
[0212]
[0213] The title compound in this step was prepared according to the method described in Example 1, Step 2, namely (2R,3R,4S,5R,6R)-2-(acetoxymethyl)-6-(2,2,2-trichloro) -1-Imineethoxy)tetrahydro-2H-pyran-3,4,5-triyltriacetate (1.5g, 3.04mmol), 2-chloro-4-nitro-phenol (634mg, 2.43 mmol), 4A molecular sieves (1.5 g) and boron trifluoride ether (0.475 mL, 3.66 mmol) were prepared by reaction in dichloromethane (24 mL), and the crude product was subjected to silica gel column chromatography (petroleum ether / ethyl acetate (v / v)=2 / 1) Purification gave the title compound as a white solid (1.0 g, 65.2%).
[0214] MS(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com