Combination therapies for the treatment of amyotropic lateral sclerosis and related disorders
A technology for diseases and diseases, which is applied in the field of combination therapy for the treatment of amyotrophic lateral sclerosis and related diseases, and can solve the problems of no microglia and no efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0163] method
[0164] chemical reagent
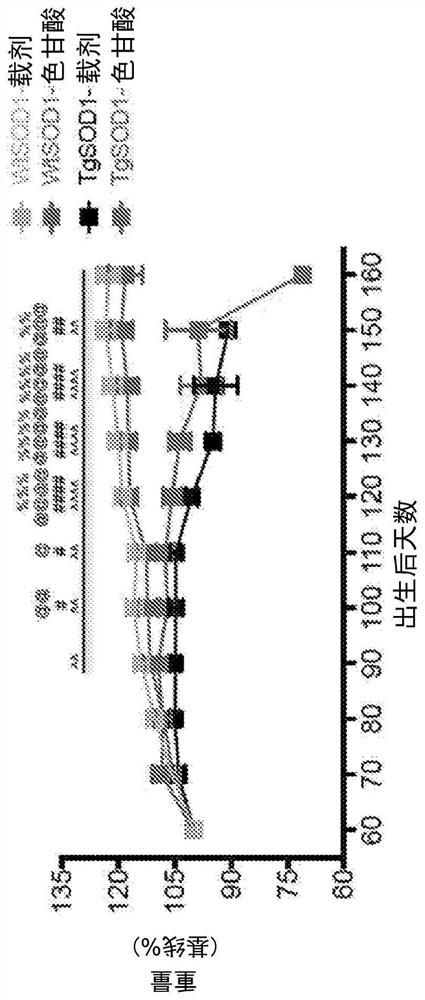
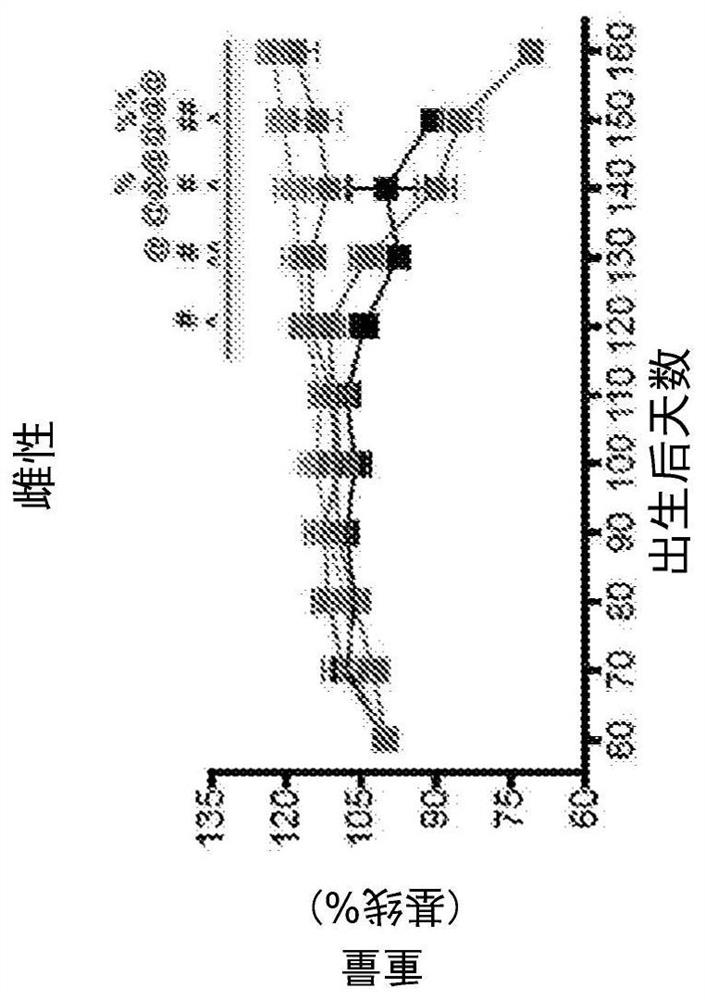
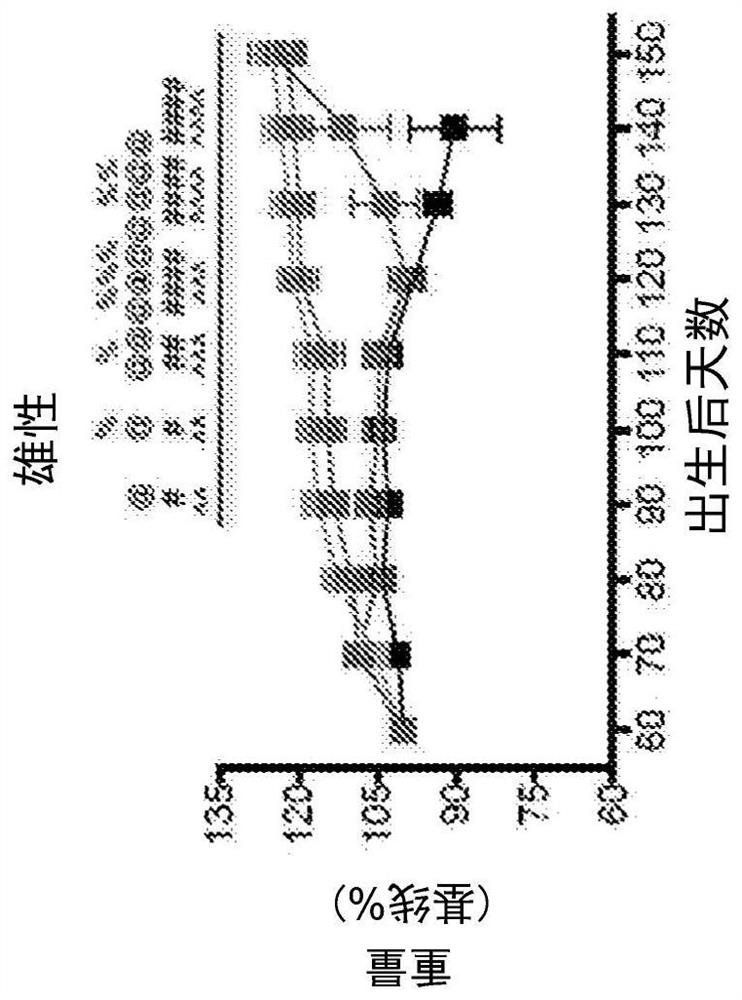
[0165] Sodium cromolyn was supplied by AZTherapies, dissolved in PBS. A 100 mM solution was used for in vivo experiments. Dulbecco's PBS was used to dilute the solution for intraperitoneal injection as previously described, with a final dose of 6.3 mg / kg.
[0166] animal
[0167] 149 mice were used in this study. All animal care, husbandry, and experiments were performed in accordance with guidelines established by the Massachusetts General Hospital Subcommittee on Research Animal Care. These experiments were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee (2014N000018). All mice had free access to food and water. Mice were regularly assessed for dyskinesia and euthanized at the onset of severe paralysis (neurological score = 4, see Neurological Score below) to minimize distress, as previously described. Mice exhibiting mild paralysis (neurological score = 2) were fitted with water bo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com