A gene detection method and application of nucleic acid mass spectrometry-based drug for rheumatic immune diseases
A technology for rheumatic immunity and genetic testing, which is applied in biochemical equipment and methods, microbiological determination/inspection, DNA/RNA fragments, etc., can solve the problem of inability to accurately and comprehensively evaluate the effectiveness and safety of treatment, different detection methods, difficult To meet the actual clinical needs and other issues, to achieve the effect of high commercial return, low cost and short cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
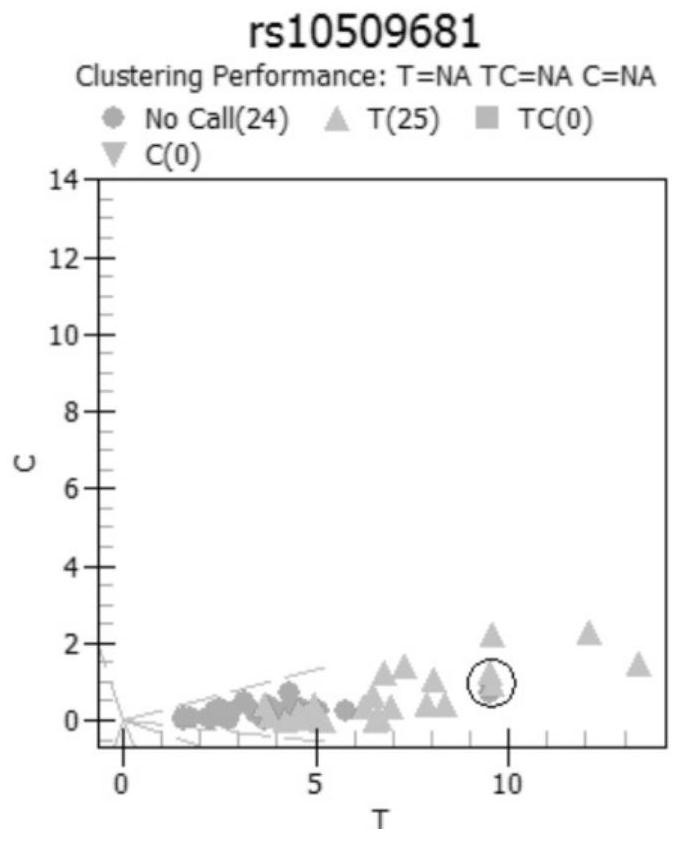
[0067] Example 1 Demonstration and Screening of Detection Sites
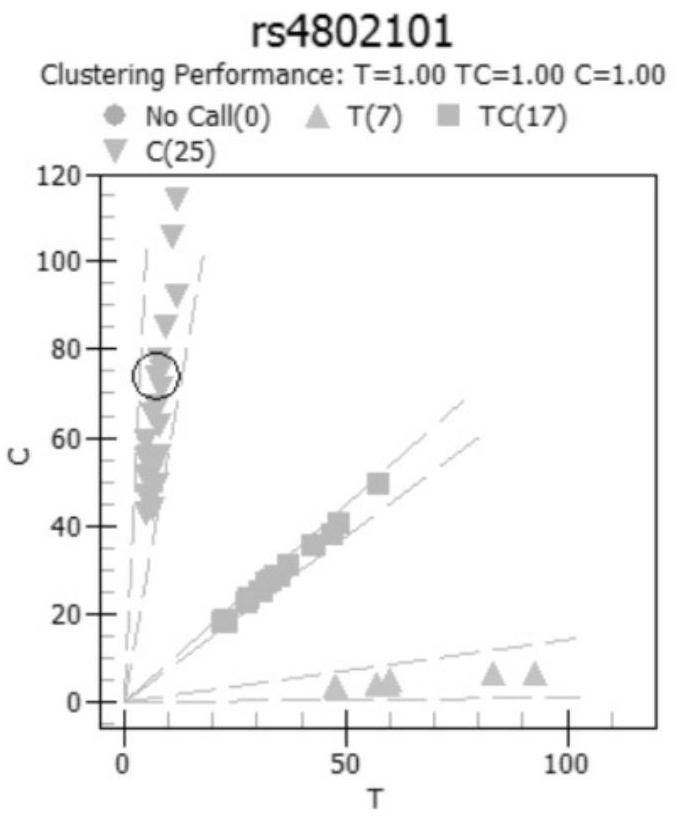
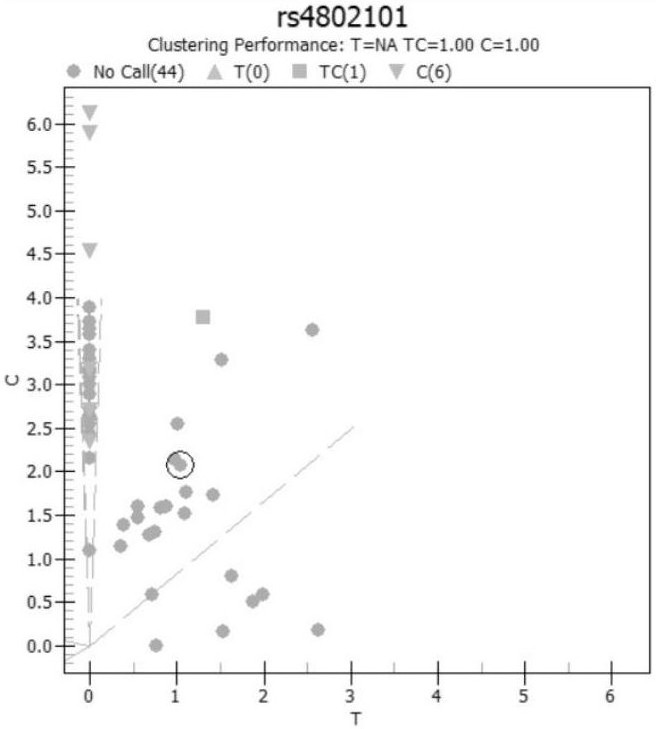
[0068] Considering the genotype characteristics of the Chinese population, the corresponding polymorphic sites for rheumatic immune diseases (especially rheumatoid) drugs were selected, and the feasible corresponding SNP sites were established as follows: rs10306114, rs1042597, rs1045642, rs1050828, rs1050829, rs10509681, rs1051266 、rs1057910、rs1061631、rs1065852、rs10919563、rs11188072、rs1128503、rs1142345、rs11572080、rs11678615、rs116855232、rs11706052、rs12041331、rs12208357、rs12248560、rs1229984、rs1495741、rs1544105、rs16947、rs1695、rs17602729、rs1799724、rs1799971、rs1800462、rs1800629、rs1801131 、rs1801133、rs1801279、rs1801280、rs2032582、rs2187668、rs2231142、rs2234693、rs2740574、rs289714、rs3131003、rs3213422、rs3397、rs34650714、rs35167514、rs361525、rs3731722、rs3758149、rs3794271、rs41303343、rs4244285、rs4646437、rs4673993、rs4802101、rs4846051、rs4986893 、rs5030865、rs5918、rs6028945、rs6138150、rs662、rs671、rs7046653、rs719235、rs7254579、rs7300...
Embodiment 2
[0069] The design of embodiment 2 primers and the establishment of reaction system
[0070] Given that MassARRAY detection is a reaction based on multiplex PCR amplification, primer combinations must avoid issues such as cross-amplification, biased amplification, and non-specificity (D. van den Boom et al. / International Journal of Mass Spectrometry, 238(2004) , 173–188), so the design of primers for this reaction system needs a lot of optimization.
[0071] First, the present invention adjusts relevant parameters through the primer design software (Assay Design Suite) of the MassARRAY website, completes the preliminary design of primers for PCR and UEP at 77 sites, derives the designed primers and each parameter file, and synthesizes the primers. Prepare amplification primer MIX and extension primer MIX according to the primer configuration table, and fine-tune the extension primer MIX until it meets the requirements. Primer testing and optimization were then performed. Spec...
Embodiment 3
[0123] Example 3 Clinical outcome experiment (compared with generation sequencing)
[0124] After confirming the optimal reaction system, the present invention carries out a series of verification experiments, including the experimental verification of accuracy, repeatability and success rate of one experiment. The specific verification scheme is as follows:
[0125] (1) Accuracy experimental verification plan: at least 5 samples from each of the 77 sites were selected for Sanger sequencing, and the results of Sanger sequencing and MassARRAY were compared.
[0126] If the consistency is required to be greater than 95%, the verification is passed.
[0127] (2) Reproducibility experiment verification scheme: 5 clinical samples were selected, and each sample was repeated twice for detection.
[0128] The consistency of the two test results is required to be 100%.
[0129] The specific verification process is as follows: firstly, the amplification primer MIX and the extension p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com