Microfluidic-technology-based CTC protein typing kit
A microfluidic technology, protein technology, applied in the field of medicine and biology, CTC protein typing kits, can solve the problems of affecting typing identification, damage cell activity, low recovery rate, etc., to achieve rapid automatic detection and typing, The effect of reducing steric hindrance and improving recycling efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
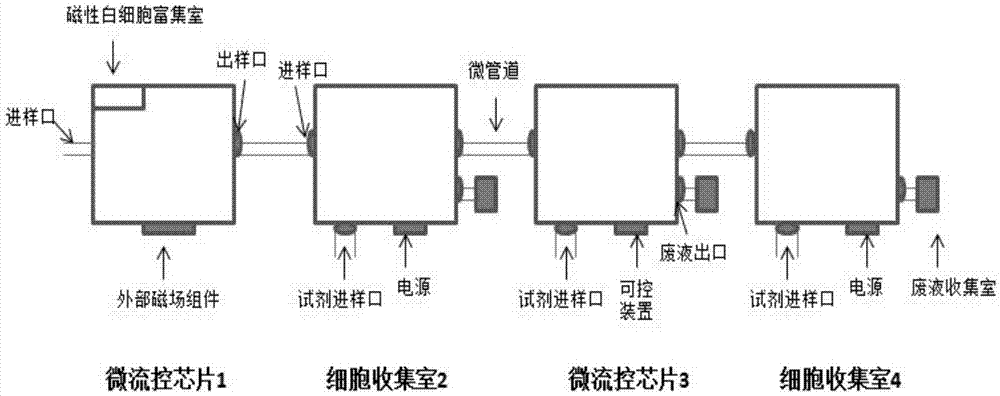
[0032] The CTC protein typing kit based on microfluidic technology described in this embodiment mainly includes:
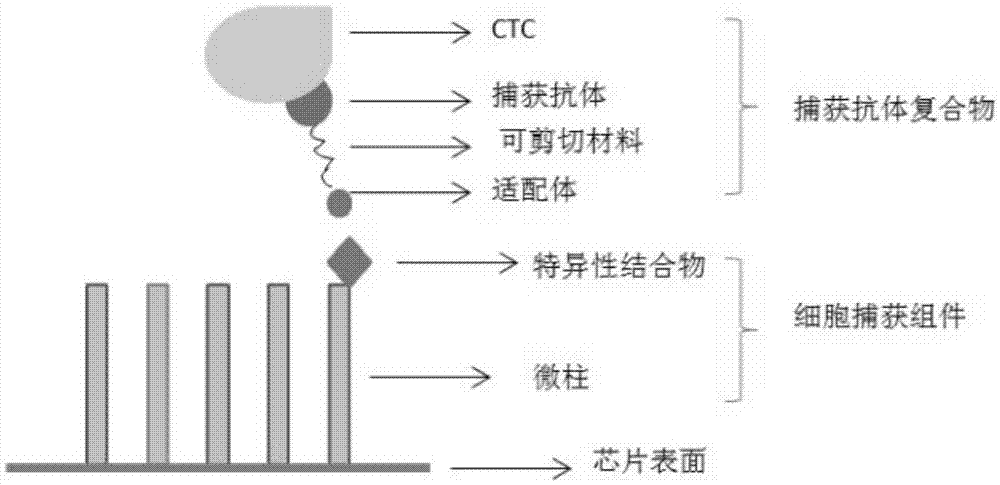
[0033] One, capture antibody complex
[0034] The capture antibody complex includes a capture antibody and an aptamer that specifically bind to each marker protein of CTCs. The capture antibody is used to connect the marker protein and the marker antibody, and can specifically bind to the marker protein and the marker antibody. The capture antibody In order to have high-purity monoclonal antibodies, the reaction characteristics of the selected capture antibodies do not interfere with each other. The capture antibody and the aptamer are connected by a shearable material. The shearable materials include photosensitive materials, temperature-sensitive materials, and humidity-sensitive materials. Materials, heat-sensitive materials, enzymatic degradation materials, etc., aptamers are small molecular compounds that can react with specific binding substances on the cell capt...
Embodiment 2
[0059] Example 2 Using the kit in Example 1 to detect circulating tumor cells in human peripheral blood
[0060] The formulations of the various solutions are as follows:
[0061]
[0062] The capture mixture and the color-developing mixture in this example both use all the antibodies in the corresponding marker protein list in Example 1.
[0063] 1. Sample pretreatment
[0064] 1. Use the preservation solution to store the blood sample in the sample preservation tube, centrifuge at 600×g for 5 min, discard the supernatant, remove the red blood cells, and add 1mL PBS to resuspend.
[0065] 2. Take 450uL of the corresponding magnetic beads and mix, centrifuge to remove the supernatant, add 1mLPBS to resuspend, use 1mg magnetic beads per 30ug magnetic bead antibody, add the magnetic bead antibody mixture to the cell suspension, and react on the mixer for 30min. Centrifuge to remove the supernatant, add 10uLPBS to resuspend.
[0066] 2. Microfluidic device detection
[0067] 1. Add the cell...
Embodiment 3
[0095] Example 3: Using the kit in Example 1 to detect tumor cell lines
[0096] 1. Selection of tumor cell lines
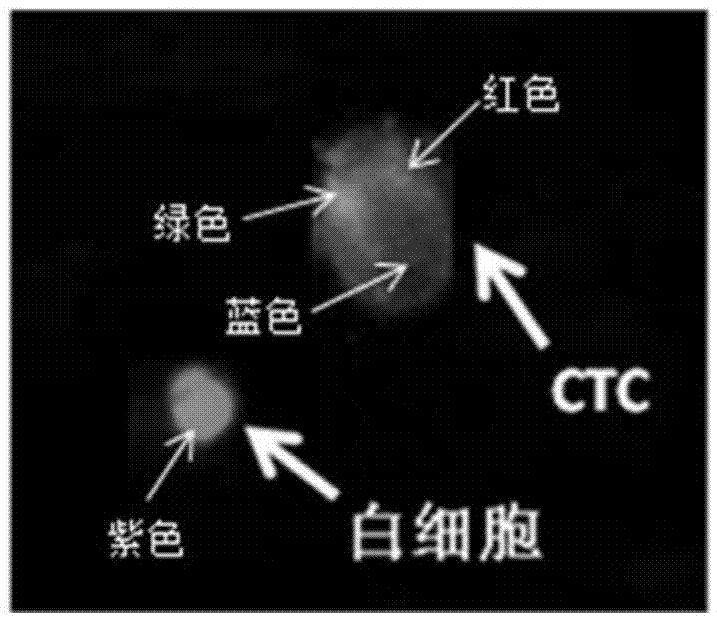
[0097] In this example, we added a known number of cells from the epithelial cell line MCF-10A, the mesenchymal tumor cell line U118, and the epithelial-mesenchymal mixed lung cancer cell line PC-9 to the peripheral blood samples of healthy volunteers, which used The lymphoblast cell line CCRF-HSB-2 was used as a negative control. Those skilled in the art can obtain it through purchase as long as they know the name of the cell line. The kit in Example 1 was used to test the tumor cell capture efficiency of the present invention.
[0098] 2. Sample detection
[0099] Take each of 200 MCF-10A cells, U118 cells and PC-9 cells (determined by a cell counter), add them to 5 mL of healthy volunteers' peripheral blood samples, mix them, and test the samples according to the detection process and method described in Example 2. , The target cell capture rate is calculated by th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com