Stabilized albumin-free recombinant factor VIII preparation having low sugar content
An albumin-stabilizing technology, applied in the field of albumin-free lyophilized preparations, which can solve problems such as destabilization effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Contains 150mM NaCl, 2.5mM CaCl by titration 2 The effect of histidine on the stability of lyophilized rF VIII was studied by using different contents of histidine in rF VIII mixture with 165mM mannitol. The results are shown in Table 1 below.
[0041] Table 1
[0042] Histidine (mM)
[0043] It can be seen from the above data that increasing the amount of histidine leads to a decrease in the efficacy of replicating lyophilized rF VIII according to the dose-determining relationship. This result suggests that histidine does not play a role in stabilizing FVIII in the lyophilized state.
Embodiment 2
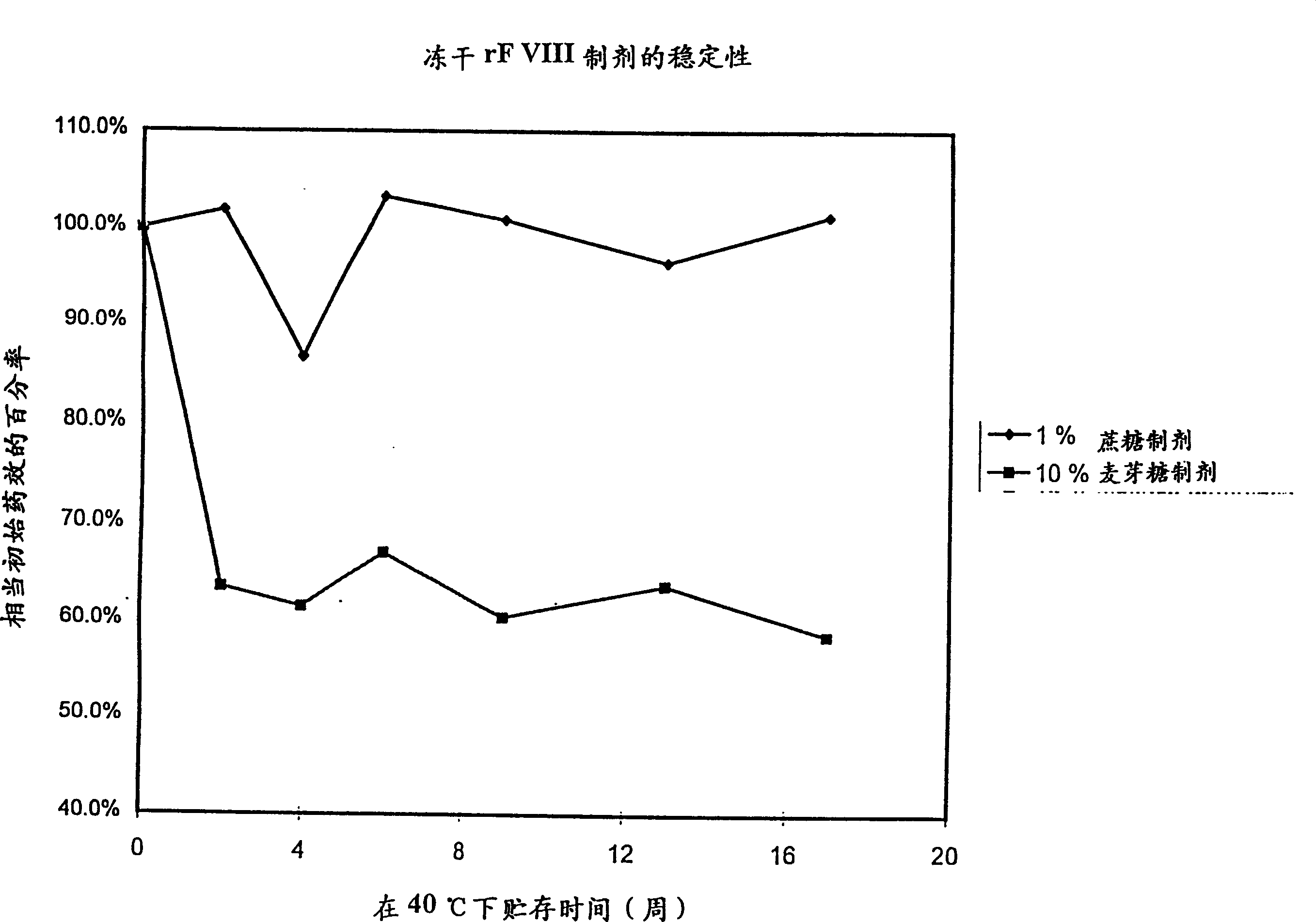
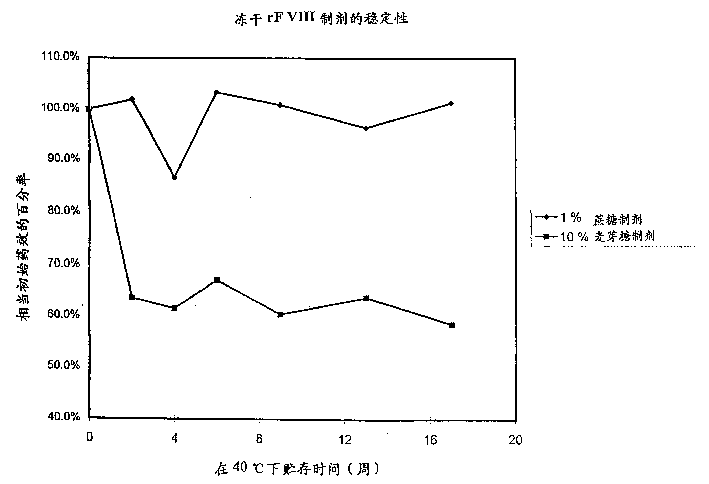
[0045] Stability of rF VIII in high-sugar and low-sugar formulations.
[0046] Recombinant coagulation factor VIII was prepared in two formulations. The instability was studied under accelerated storage conditions at 40°C.
[0047] Similar to the prior art formulations, the high sugar formulation is an amorphous formulation containing 50 mM sodium chloride, 2.5 mM calcium chloride, 5 mM histidine and 10% by weight maltose after reconstitution in water.
[0048] The low sugar-containing formulation of the present invention is a lens comprising an amorphous component of 1% sucrose (30 mM sucrose) for stabilizing the protein. After replication with WFI, the preparation contained 30 mM sodium chloride, 2.5 mM calcium chloride, 20 mM histidine, 290 mM glycine and approximately 200 IU / ml rF VIII. This formulation is compared with the prior art formulation in the accompanying drawings, where it can be seen that the low sugar rF VIII formulation of the present invention is more stab...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com