Preparation method of high-purity p-nitrochlorobenzene
A technology of p-nitrochlorobenzene and nitrochlorobenzene, which is applied in the field of high-purity p-nitrochlorobenzene preparation, can solve the problems of restricting the quality of aminoanisole products and low purity of p-nitrochlorobenzene, and meet the needs of the market Demand, the effect of increasing economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
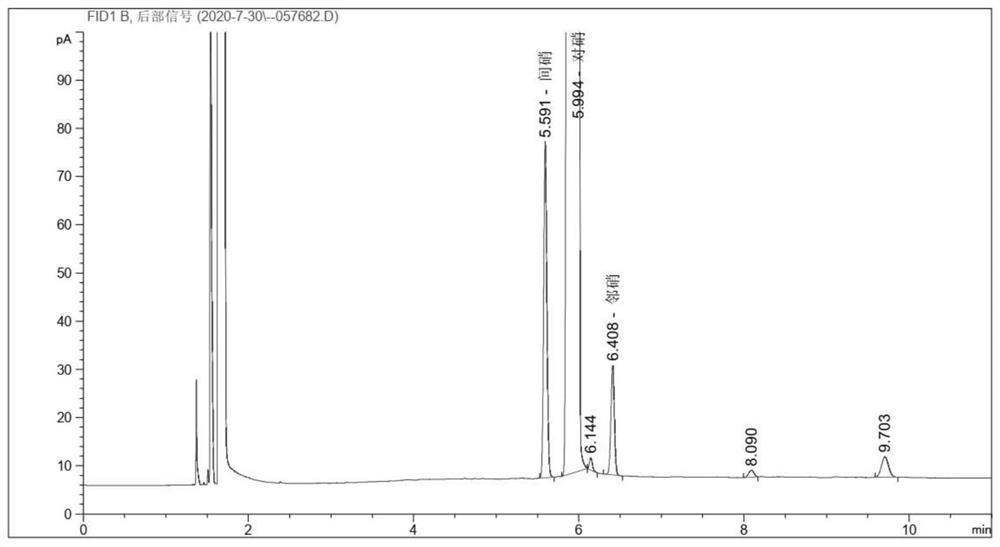
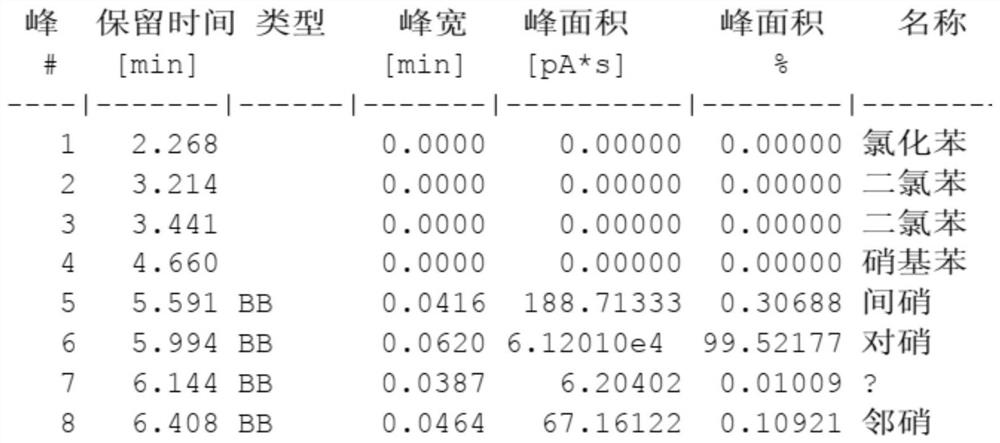
[0037] In a specific embodiment, a method for preparing high-purity p-nitrochlorobenzene comprises the following steps: after nitric acid and sulfuric acid are mixed into a mixed acid, and then carry out nitration reaction with chlorobenzene to generate nitrochlorobenzene; The neutral nitrochlorobenzene is obtained through the steps of oleic acid separation, alkali washing and water washing; after the neutral nitrochlorobenzene is dried, the finished p-nitrochlorobenzene is separated by a crystallization process.
[0038] It is worth noting that the above process belongs to the traditional production process of p-nitrochlorobenzene, and its reaction control conditions, including parameters such as reaction material ratio, reaction temperature, reaction pressure, and space velocity, belong to those skilled in the art. Known or should know technical means, in the present invention, only improve the process of crystallization process separation p-nitrochlorobenzene, do not emphasi...
Embodiment 1
[0066] Using neutral nitrochlorobenzene as raw material, carry out primary crystallization.
[0067] After nitric acid and sulfuric acid are made into mixed acid, they are nitrated with chlorobenzene to generate nitrochlorobenzene; nitrochlorobenzene undergoes oleic acid separation, alkali washing, and water washing steps in sequence to obtain neutral nitrochlorobenzene; neutral nitrochlorobenzene After the chlorobenzene is dried, a crystallization is carried out.
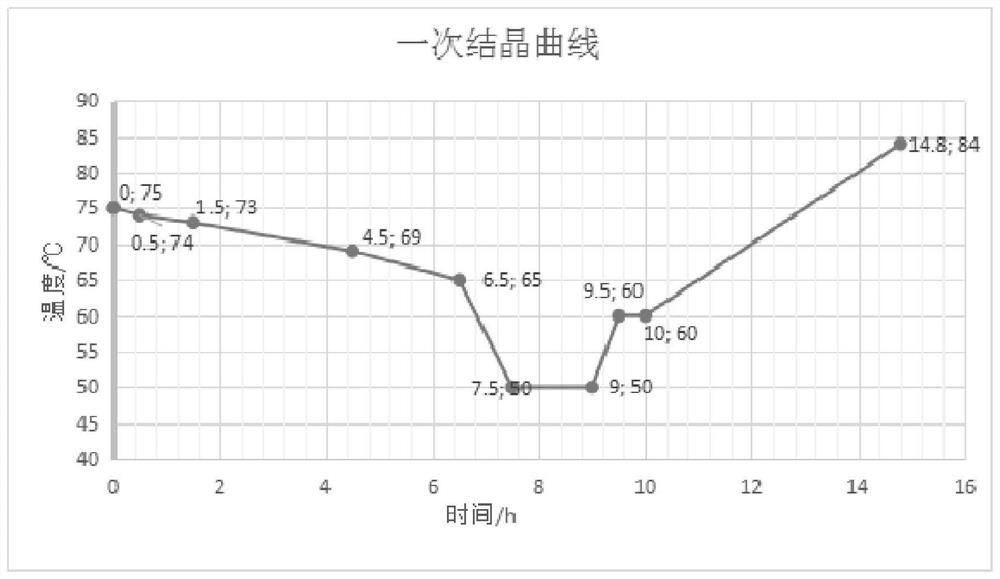
[0068] Please see figure 1 , the initial material temperature of primary crystallization is about 80±1°C, firstly lower the temperature to about 74°C, and start to strictly control the cooling rate, first reduce the temperature to about 73°C at a cooling rate of 1°C / h, and then reduce the temperature to about 1.3°C / h ~1.5°C / h cooling rate, reduce the temperature to about 69°C, and then reduce the temperature to about 65°C at a cooling rate of 2°C / h, so far the crystallization forming period of the first crystalliz...
Embodiment 2
[0074] Using neutral nitrochlorobenzene as a raw material, the primary crystallization process in Example 1 was adjusted.
[0075] The primary crystallization process in Example 1 is adjusted in order to further improve the purity of the primary crystallization p-nitrochlorobenzene. In a preferred implementation process, comprise the following steps:
[0076] Please see Figure 4 , the initial material temperature of primary crystallization is about 80±1°C, firstly cool down to about 76°C, and start to strictly control the cooling rate, firstly lower the temperature to about 74°C at a cooling rate of 1°C / h, and then reduce the temperature to about 74°C at a cooling rate of 1.3°C / h ~1.5°C / h cooling rate, lower the temperature to about 72°C, and then lower the temperature to about 68°C at a cooling rate of 2°C / h, so far the crystallization forming period of the first crystallization is completed. In this stage, the crystal nucleus grows to a certain scale and has a certain str...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com