Preparation and application of beta-cyclodextrin metal organic framework material HPLC column
A technology of cyclodextrin and organic ligand is applied in the field of preparation and application of chiral high performance liquid chromatography column materials to achieve the effect of expanding the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1: Preparation of β-CD-MOFs materials β-CD-MOFs-P50 and β-CD-MOFs-P20
[0064] One-pot synthesis of β-CD-MOFs-P50:
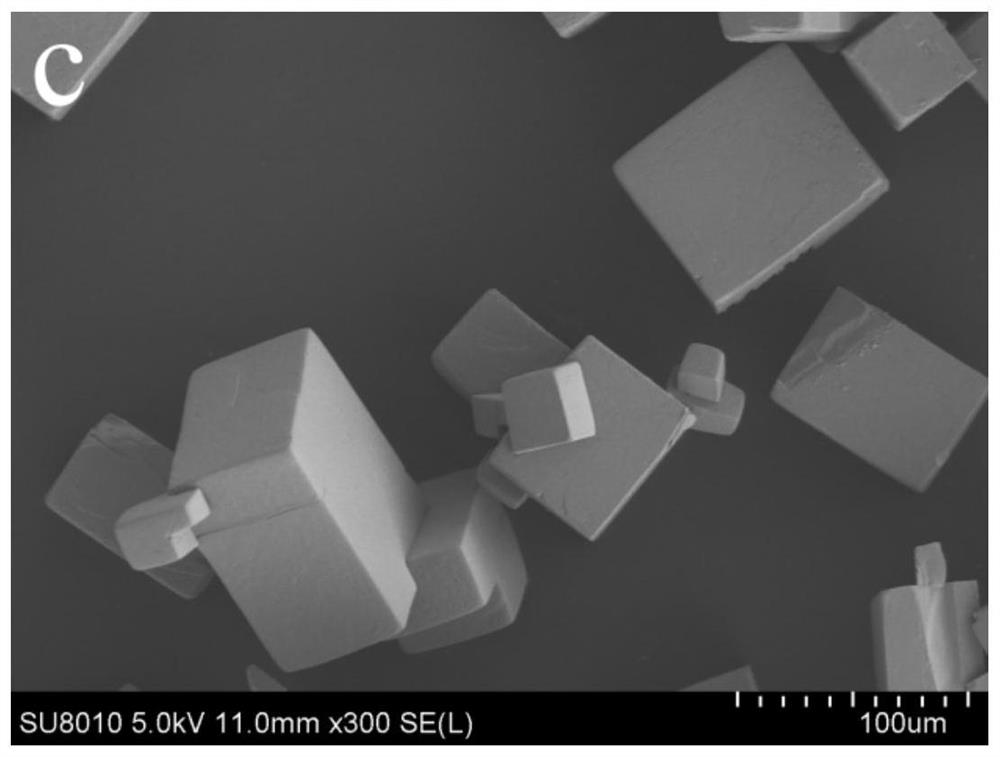
[0065] β-CD (0.67g), m-carboxybenzenesulfonyl chloride (0.52g) and potassium chloride (0.31g) were stirred and dissolved in 26mL methanol aqueous solution (concentration can be 20-90v / v%, no influence on product morphology) In this example, 120 mg of CTAB was added into methanol solution with a concentration of 60v / v%, sealed, and allowed to stand at room temperature for 24 hours to obtain colorless crystals, which were filtered and dried for later use. At this time, the particles of the obtained β-CD-MOFs material are relatively uniform, with a particle size of about 50 μm, named β-CD-MOFs-P50, and its specific appearance is as follows figure 1 shown.
[0066] Synthesis of β-CD-MOFs-P20 by vapor diffusion method:
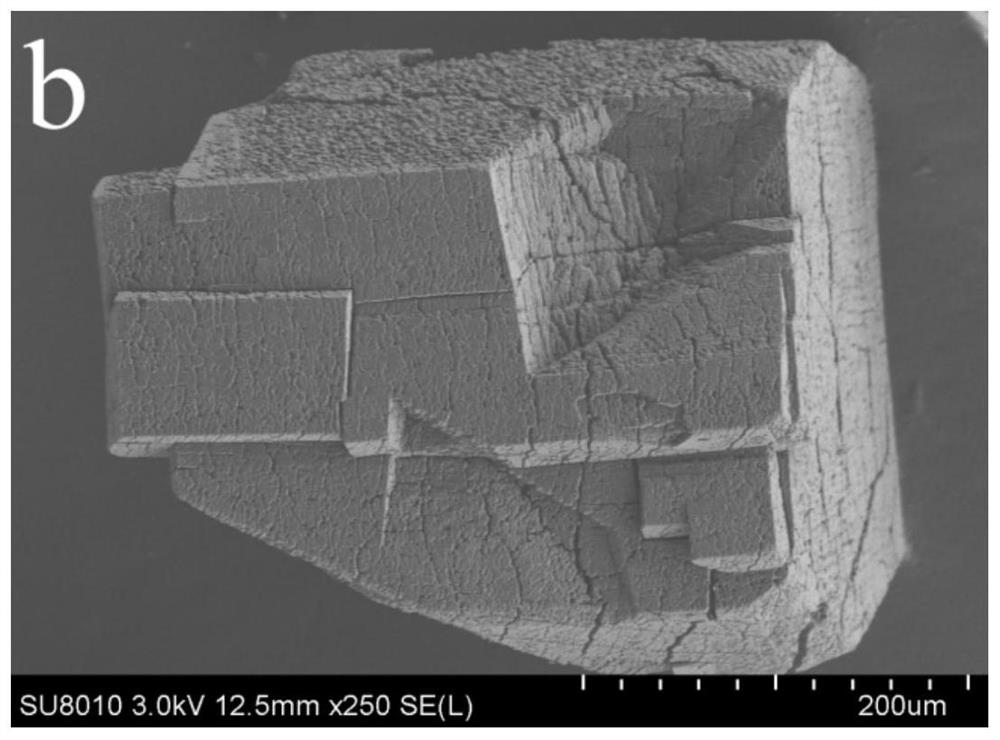
[0067] β-CD (0.1000g), m-carboxybenzenesulfonyl chloride (0.0778g) and potassium chloride (0.0460g) were stirred and dissolved in ...
Embodiment 2
[0075] Example 2 Preparation of β-CD-MOFs chiral HPLC column
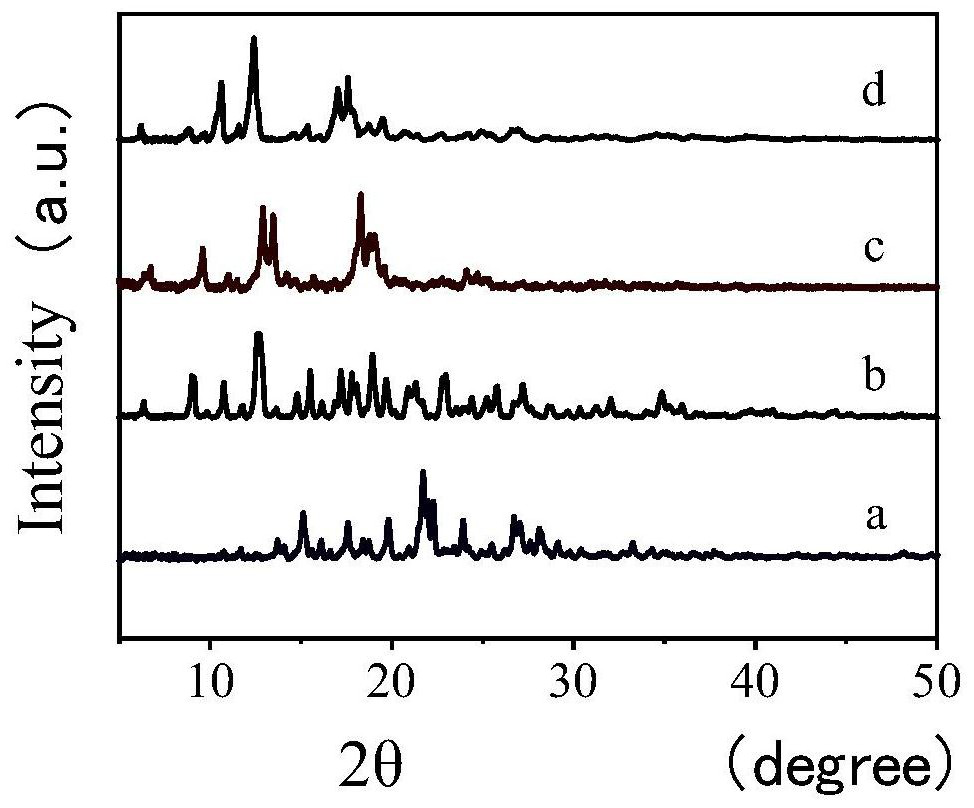
[0076] Using the two β-CD-MOFs materials synthesized in Example 1 as chiral separation materials, they were prepared as chiral HPLC column fillers, and then prepared high-performance liquid chromatography HPLC columns. Transmission electron microscopy and scanning electron microscopy were used to confirm the properties of the fillers. Pros and cons.
[0077] 1. Preparation method of HPLC column packing
[0078] The preparation method is as follows: first acidify commercially available silica beads with a particle size of 5 μm (i.e. fully porous spherical silica gel), dry them for later use; β-CD-MOFs (β-CD-MOFs-P50 or β-CD-MOFs-P20) in N 2 In the atmosphere, add the treated fully porous spherical silica gel (the mass ratio of β-CD-MOFs to fully porous spherical silica gel in this example is 1:2), stir and react at 115°C for 24h, filter, wash and dry after the reaction is complete ,spare.
[0079] 2. Characteriz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap