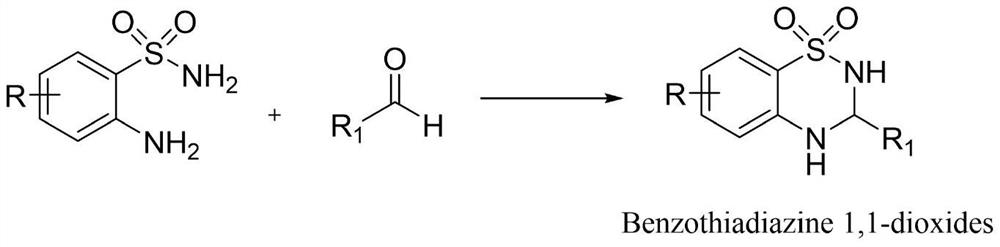
Preparation method of benzothiadiazine-1, 1-dioxide compound
A technology of benzothiadiazine and dioxide, which is applied in the field of synthesis of benzothiadiazine-1,1-dioxide compounds, and can solve problems affecting the application of active raw materials, low-efficiency pollution, cumbersome operations, etc. problems, to achieve the effect of eliminating heavy metal pollution, reducing the use of toxic solvents, and improving product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A kind of synthetic method of benzothiadiazine-1,1-dioxide compound, the steps are as follows:
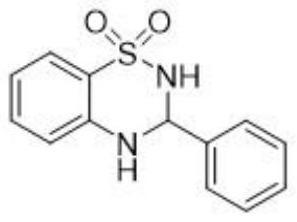
[0022] Add 2-aminobenzenesulfonamide (1.0g, 5.80mmol) and benzaldehyde (0.62g, 5.80mmol) into a 25mL round-bottomed flask, heat up to 60°C, and react for 1 hour with the open door open, and monitor the reaction progress with TLC. After the reaction is complete, it is directly separated and dried to obtain off-white 3-phenyl-1,2,4-benzothiadiazine-1,1-dioxide powder. The yield was 98.2%, and the purity was 99.8%.
[0023]
[0024] The product was tested: m.p.: 129–132°C; 1 H NMR (400MHz, DMSO) δ7.90(s,1H),7.72–7.63(m,2H),7.53(d,J=7.2Hz,1H),7.51–7.43(m,3H),7.42(s, 1H), 7.36–7.28(m, 1H), 6.92(d, J=8.3Hz, 1H), 6.77(t, J=7.5Hz, 1H), 5.79(s, 1H); 13 C NMR (100MHz, DMSO) δ144.40, 137.79, 133.33, 129.63, 129.02, 128.06, 124.24, 122.12, 117.22, 116.88, 68.89.
Embodiment 2
[0026] A kind of synthetic method of benzothiadiazine-1,1-dioxide compound, the steps are as follows:
[0027] Add 2-aminobenzenesulfonamide (1.0g, 5.80mmol) and p-tolualdehyde (0.70g, 5.80mmol) into a 25mL round-bottomed flask, heat up to 60°C, and react for 1 hour with the open door open, and monitor the reaction progress by TLC. After the reaction is complete, directly separate and dry to obtain off-white 3-(4-methylphenyl)-1,2,4-benzothiadiazine-1,1-dioxide powder. The yield was 98.2%, and the purity was 96.2%.
[0028]
[0029] The product was tested: m.p.: 153–154°C; 1 HNMR (400MHz, DMSO) δ7.85 (d, J = 11.8Hz, 1H), 7.53 (t, J = 8.7Hz, 3H), 7.42–7.20 (m, 4H), 6.90 (d, J = 8.3Hz, 1H), 6.76(t, J=7.5Hz, 1H), 5.73(d, J=11.7Hz, 1H), 2.35(s, 3H); 13 C NMR (100MHz, DMSO) δ144.40, 139.00, 134.93, 133.28, 129.47, 127.93, 124.22, 122.05, 117.14, 116.84, 68.67, 21.30.
Embodiment 3
[0031] A kind of synthetic method of benzothiadiazine-1,1-dioxide compound, the steps are as follows:
[0032] Add 2-aminobenzenesulfonamide (1.0g, 5.80mmol) and p-fluorobenzaldehyde (0.72g, 5.80mmol) into a 25mL round-bottomed flask, heat up to 60°C, and react for 1 hour with the open door open, and monitor the reaction progress by TLC. After the reaction is complete, it is separated directly and dried to obtain white 3-(4-fluorophenyl)-1,2,4-benzothiadiazine-1,1-dioxide powder. The yield was 98.3%, and the purity was 99.8%.
[0033]
[0034] The product was tested: m.p.: 176-178°C; 1 H NMR (400MHz, DMSO) δ7.92, J = 11.9Hz, 1H), 7.73 (dd, J = 8.7, 5.5Hz, 2H), 7.58–7.50 (m, 1H), 7.41 (s, 1H), 7.32 (ddd, J=15.1, 7.3, 4.1Hz, 3H), 6.90(d, J=8.0Hz, 1H), 6.82–6.71(m, 1H), 5.81(d, J=11.8Hz, 1H); 13 C NMR (100MHz, DMSO) δ164.31, 161.87, 144.51, 134.39, 134.36, 133.56, 130.58, 130.49, 124.44, 122.33, 117.52, 117.06, 116.11, 115.90, 68.34.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com