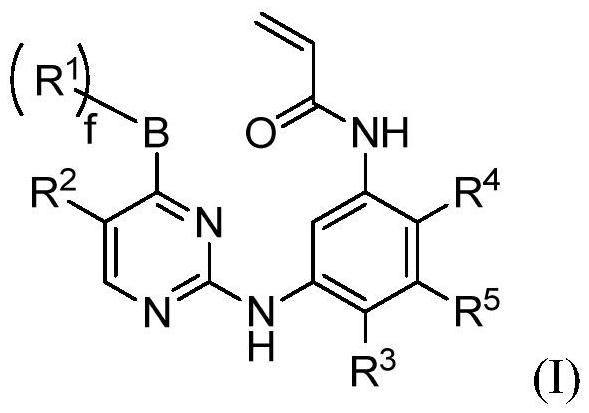
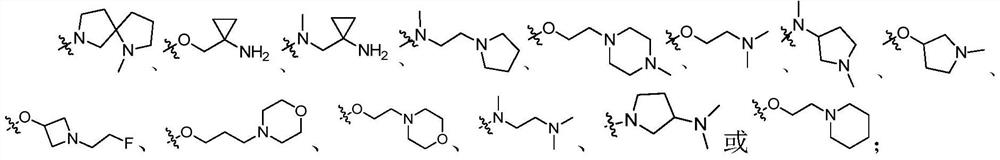
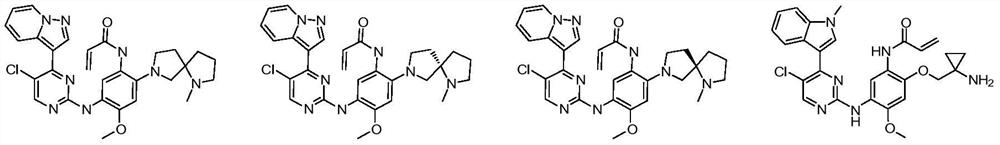
(Substituted phenyl) (substituted pyrimidine) amino derivative as well as preparation method and pharmaceutical application thereof
A technology of substituents and alkyl groups, which is applied in the field of preparation of tumor drugs, can solve the problems of limited clinical effectiveness of drugs, rashes and diarrhea in patients, and restrictions on the dosage of second-generation EGFR inhibitors, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0402] N-(5-((5-chloro-4-(pyrazolo[1,5-a]pyridin-3-yl)pyrimidin-2-yl)amino)-4-methoxy-2-(1- Methyl-1,7-diazaspiro[4.4]nonan-7-yl)phenyl)acrylamide (compound 1)
[0403] N-(5-((5-chloro-4-(pyrazolo[1,5-a]pyridin-3-yl)pyrimidin-2-yl)amino)-4-methoxy-2-(1-methyl-1, 7-diazaspiro[4.4]nonan-7-yl)phenyl)acrylamide
[0404]
[0405]
[0406] The first step: 5-((5-chloro-4-(pyrazolo[1,5-a]pyridin-3-yl)pyrimidin-2-yl)amino)-4-methoxy-2-(1 -Methyl-1,7-diazaspiro[4.4]nonan-7-yl)-1-nitrobenzene (1B)
[0407] 5-((5-chloro-4-(pyrazolo[1,5-a]pyridin-3-yl)pyrimidin-2-yl)amino)-4-methoxy-2-(1-methyl-1,7-diazaspiro [4.4]nonan-7-yl)-1-nitrobenzene
[0408] 5-((5-chloro-4-(pyrazolo[1,5-a]pyridin-3-yl)pyrimidin-2-yl)amino)-4-methoxy-2-fluoro-1-nitro phenylbenzene (intermediate 5) (0.3g, 0.72mmol), 1-methyl-1,7-diazaspiro[4.4]nonane (1A) (0.15g, 10.8mmol) and N,N-diiso Propylethylamine (ie DIPEA) (0.28g, 2.17mmol) was added to N,N-dimethylformamide (3mL), and microwaved at 140°C for 1.5 ...
Embodiment 2
[0422] (S)-N-(5-((5-chloro-4-(pyrazolo[1,5-a]pyridin-3-yl)pyrimidin-2-yl)amino)-4-methoxy-2 -(1-Methyl-1,7-diazaspiro[4.4]nonan-7-yl)phenyl)acrylamide (compound 2)
[0423] (S)-N-(5-((5-chloro-4-(pyrazolo[1,5-a]pyridin-3-yl)pyrimidin-2-yl)amino)-4-methoxy-2-(1- methyl-1,7-diazaspiro[4.4]nonan-7-yl)phenyl)acrylamide
[0424]
Embodiment 3
[0426](R)-N-(5-((5-chloro-4-(pyrazolo[1,5-a]pyridin-3-yl)pyrimidin-2-yl)amino)-4-methoxy-2 -(1-Methyl-1,7-diazaspiro[4.4]nonan-7-yl)phenyl)acrylamide (Compound 3)
[0427] (R)-N-(5-((5-chloro-4-(pyrazolo[1,5-a]pyridin-3-yl)pyrimidin-2-yl)amino)-4-methoxy-2-(1- methyl-1,7-diazaspiro[4.4]nonan-7-yl)phenyl)acrylamide
[0428]
[0429] The split method of embodiment 2 and embodiment 3 is as follows:
[0430] Take N-(5-((5-chloro-4-(pyrazolo[1,5-a]pyridin-3-yl)pyrimidin-2-yl)amino)-4-methoxy-2-(1 -Methyl-1, 7-diazaspiro[4.4]nonan-7-yl)phenyl)acrylamide (compound 1) (100mg) is used for resolution, and the preparation conditions are: Instrument GX-281C0630, (0.1 %diethylamine+n-hexane) / isopropanol=70 / 30, 10.0mL / min, 254nm, column temperature 40°C (AD-H 20*250mm 5um H-37); two optical isomers were obtained: iso Conformant 1 (peak 1, 50mg, yellow solid, ee%=100%, t=33.97min), isomer 2 (peak 2, 50mg, yellow solid, ee%=100%, t=40.78min).
[0431] Isomer 1
[0432] 1 H NMR (400M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com