Immune cell in-vitro induction amplification, cryopreservation and resuscitation method
An immune cell and cryopreservation technology, applied in the field of immune cell expansion in vitro, can solve the problems of low cell growth efficiency, poor NK cell performance, and high production cost, and achieve the goal of promoting immune cell expansion, improving activation efficiency, and ensuring efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
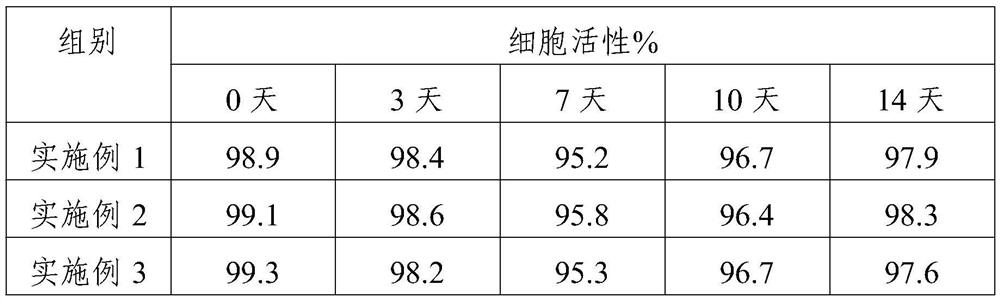
[0040] Place the mononuclear cells in No. 1 medium for activating culture in the coated culture container for 24 hours, then use No. 4 medium, add EPO at 1.2 ng / ml after 2 hours of culture, and then culture until the differentiation of immune cells When the expression of CD56 exceeds 90%, use No. 7 medium to continue the culture, add IL-2 at 500IU / ml after the secondary expansion culture for more than 2 hours, and then continue the culture; record it as the 0th day, observe the cells every day, according to the culture Change the medium every 2-3 days to subculture once.
Embodiment 2
[0042] Place the mononuclear cells in No. 2 medium for activation and culture in the coated culture container for 24 hours, then use No. 5 medium, add EPO at 2.0 ng / ml after 2 hours of culture, and then culture until the differentiation of immune cells When the expression of CD56 exceeds 90%, use No. 8 medium to continue the culture, add IL-2 at 600IU / ml after the secondary expansion culture for more than 2 hours, and then continue the culture; record it as the 0th day, observe the cells every day, according to the culture Change the medium every 2-3 days to subculture once.
Embodiment 3
[0044] Place the mononuclear cells in No. 3 medium for activation and culture in the coated culture container for 24 hours, then use No. 6 medium, add EPO at 1.6 ng / ml after 2 hours of culture, and then culture until the differentiation of immune cells When the expression of CD56 exceeds 90%, use No. 9 medium to continue culturing, add IL-2 at 550IU / ml after the secondary expansion culture for more than 2 hours, and then continue culturing; record it as day 0, observe the cells every day, according to the culture Change the medium every 2-3 days to subculture once.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com