Iron/cobalt duplex-metal organic framework material as well as preparation method and application thereof
An organic framework and bimetallic technology, applied in the field of iron/cobalt bimetallic organic framework materials and their preparation, can solve the problems of poor dispersion performance and diffusivity, insufficient active center sites, incomplete degradation of pollutants, etc., and achieve easy recycling Utilization, short reaction time, easy-to-achieve effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
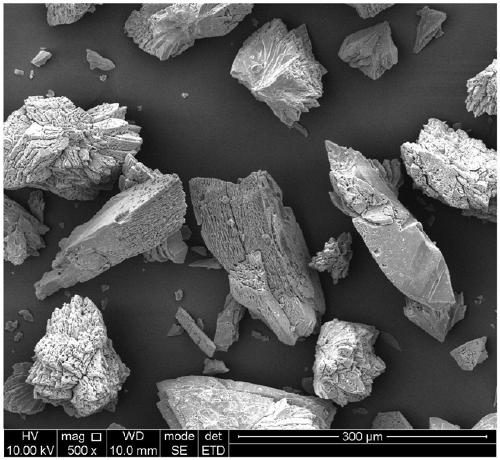
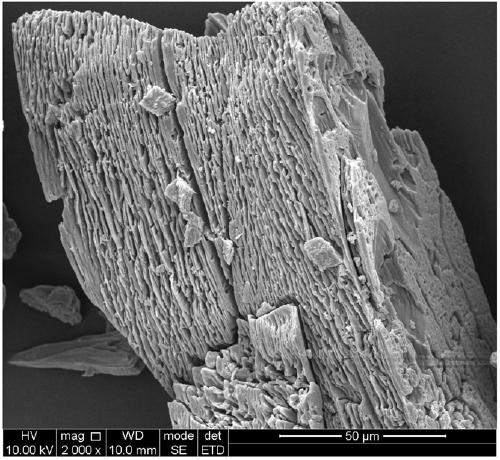
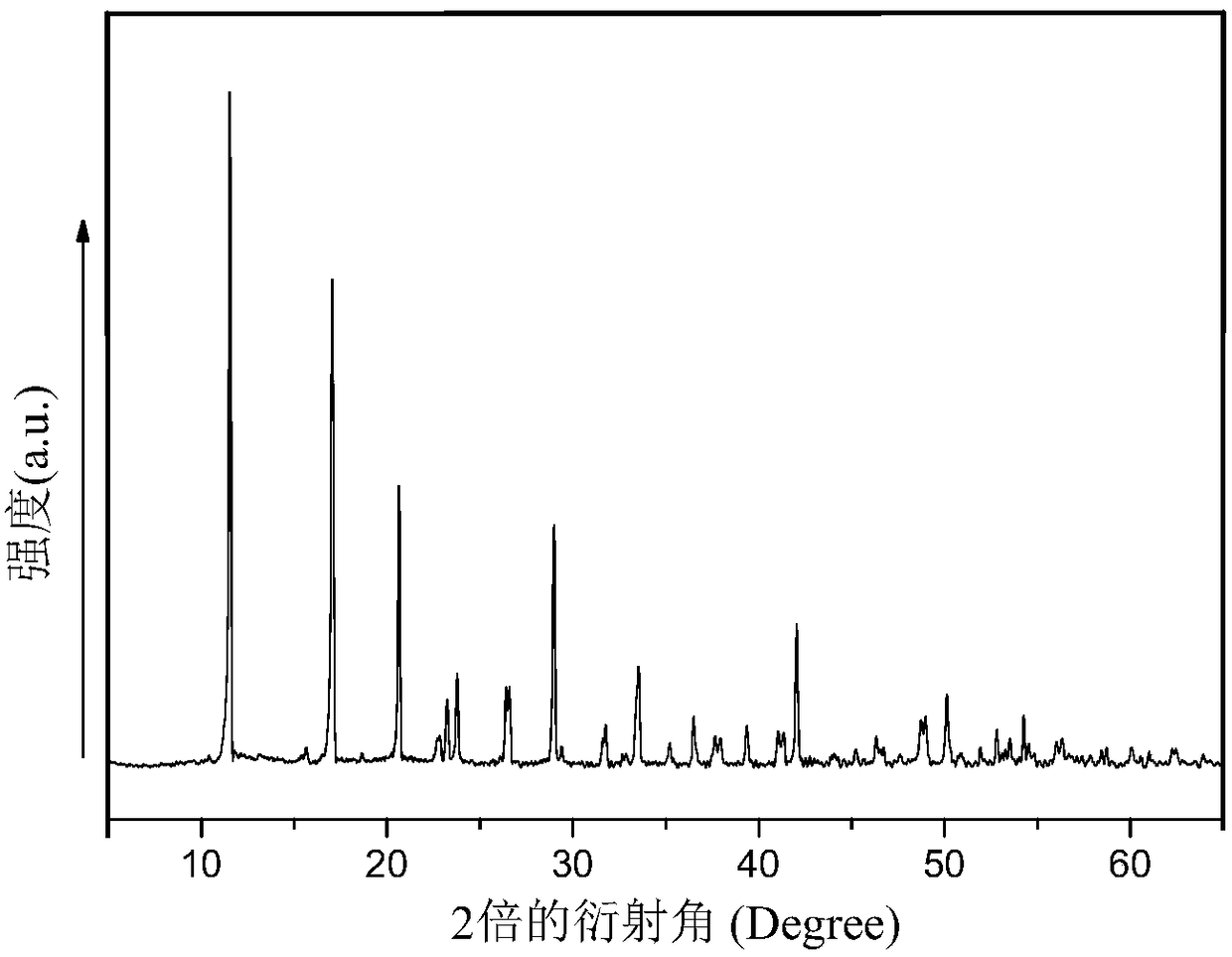
[0044] Preparation of Fe / Co Bimetallic Organic Frameworks (Fe / Co Bimetallic MOFs)
[0045] (1) Preparation of precursor solution: 1.746g (6mM) Co(NO 3 ) 2 ·6H 2 O, 1.194g FeCl 2 4H 2 O (6mM) and 1.994g (12mM) terephthalic acid (BDC) were dissolved in 60mL of N,N-dimethylformamide (DMF), and then 2.4mL of hydrofluoric acid (HF) was added, and the above mixture The solution was stirred until completely dissolved to obtain a precursor solution;
[0046] (2) Preparation of iron / cobalt bimetallic MOFs: Transfer the precursor solution prepared in step (1) to a 100mL polytetrafluoroethylene-lined autoclave, put the autoclave into a programmed oven, and heat the solvent at 150 °C Thermal reaction for 24 hours; cooling, naturally cooling down to room temperature, vacuum pump filtration, repeated washing with absolute ethanol, N, N-dimethylformamide (DMF) and deionized water to obtain a reddish-brown precipitate; put the precipitate at 100°C Dry in a vacuum oven for 12 hours to ob...
Embodiment 2
[0049] Preparation of Fe / Co Bimetallic Organic Frameworks (Fe / Co Bimetallic MOFs)
[0050] (1) Preparation of precursor solution: 2.095g (7.2mM) Co(NO 3 ) 2 ·6H 2 O, 0.954g (4.8mM) FeCl 2 4H 2 O and 1.994g (12mM) terephthalic acid (BDC) were dissolved in 60mL of N,N-dimethylformamide (DMF), then 2.4mL of hydrofluoric acid (HF) was added, and the above mixture was stirred until Completely dissolve to obtain a precursor solution;
[0051] (2) Preparation of iron / cobalt bimetallic MOFs: Transfer the precursor solution prepared in step (1) to a 100 mL polytetrafluoroethylene-lined autoclave, put the autoclave into a programmed oven, Thermal reaction for 24 hours; cooling, naturally cooling down to room temperature, vacuum pump filtration, repeated washing with absolute ethanol, N, N-dimethylformamide (DMF) and deionized water to obtain a reddish-brown precipitate; put the precipitate at 100°C Dry in a vacuum oven for 12 hours to obtain a reddish-brown solid powder, which is ...
Embodiment 3
[0054] Preparation of Fe / Co Bimetallic Organic Frameworks (Fe / Co Bimetallic MOFs)
[0055] (1) Preparation of precursor solution: 1.946g (6mM) Co(NO 3 ) 2 ·6H 2 O, 1.194g FeCl 2 4H 2 O (6mM) and 1.329g (8mM) terephthalic acid (BDC) were dissolved in 60mL of N,N-dimethylformamide (DMF), and then 2.4mL of hydrofluoric acid (HF) was added, and the above mixture The solution was stirred until completely dissolved to obtain a precursor solution;
[0056] (2) Preparation of iron / cobalt bimetallic MOFs: Transfer the precursor solution prepared in step (1) to a 100 mL polytetrafluoroethylene-lined autoclave, put the autoclave into a programmed oven, Thermal reaction for 24 hours; cooling, naturally cooling down to room temperature, vacuum pump filtration, repeated washing with absolute ethanol, N, N-dimethylformamide (DMF) and deionized water to obtain a reddish-brown precipitate; put the precipitate at 100°C Dry in a vacuum oven for 12 hours to obtain a reddish-brown solid powd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com