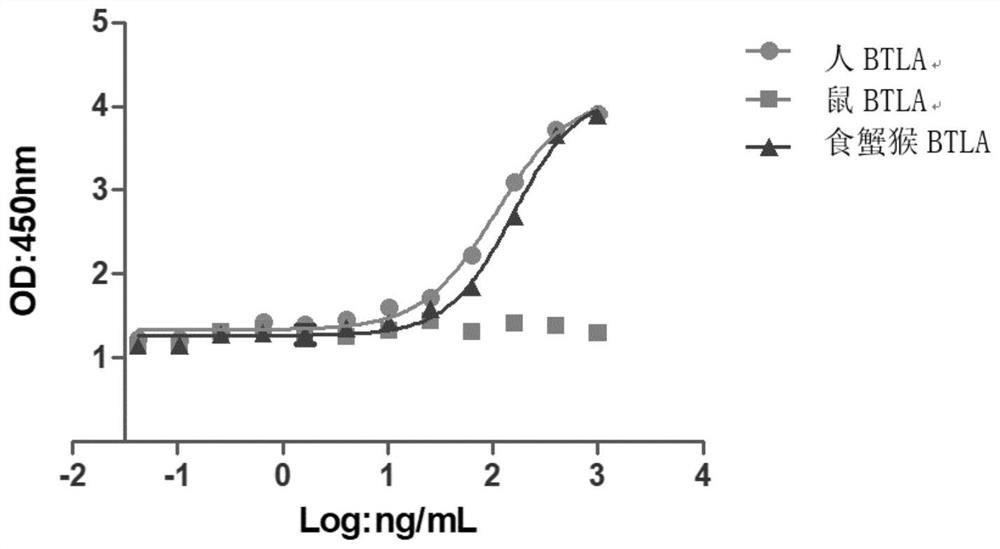
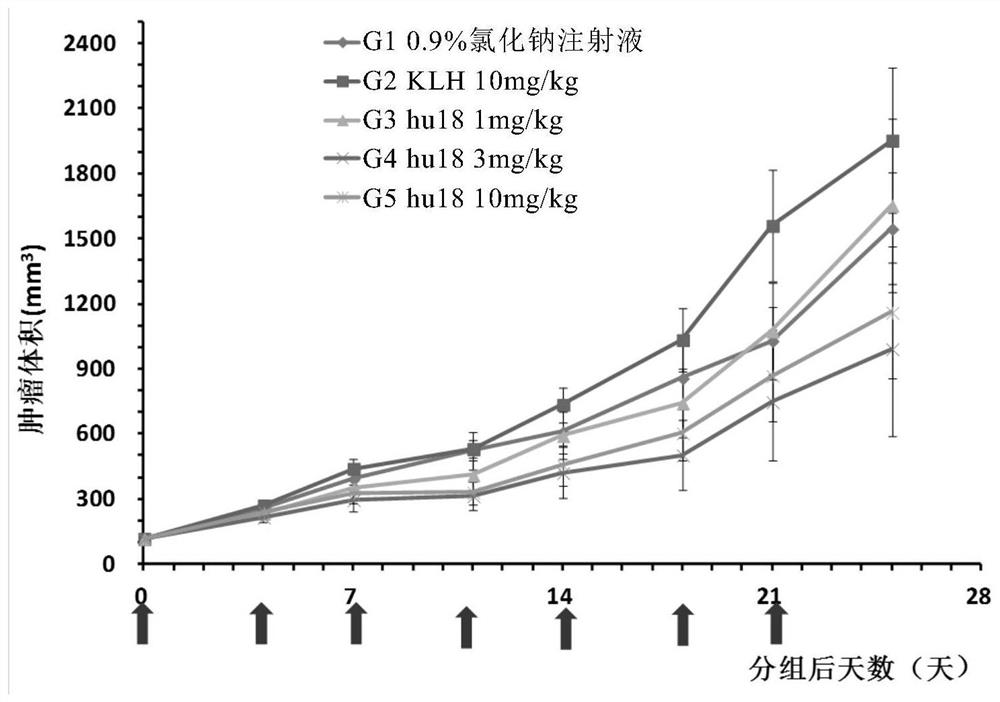
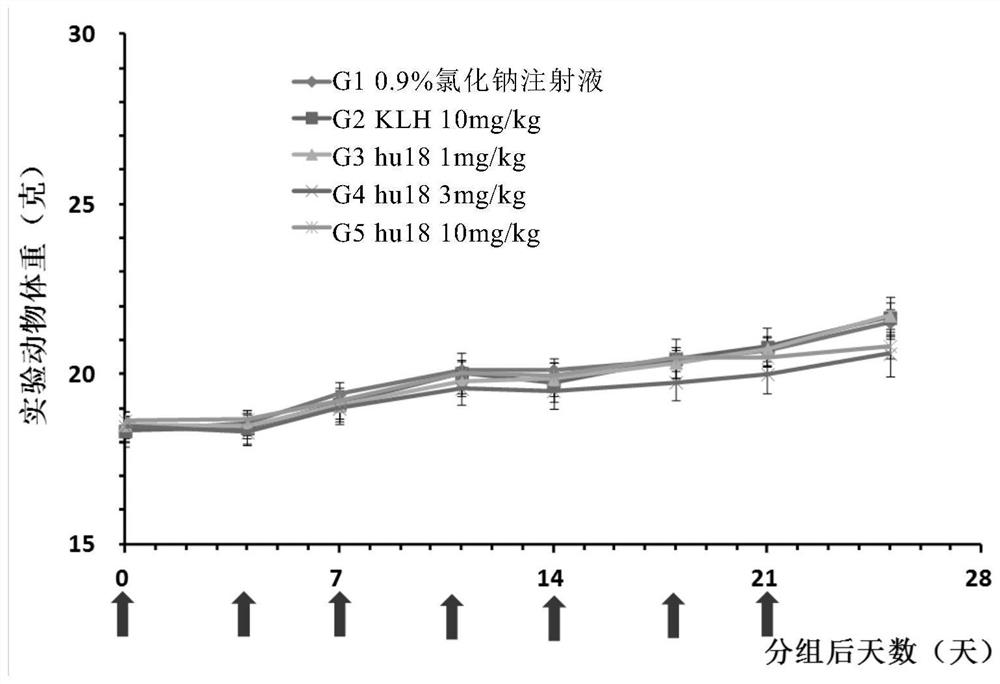
Anti-BTLA antibody pharmaceutical composition and application thereof
A composition and drug technology, applied in the direction of drug combination, antibody, drug delivery, etc., can solve problems such as predictive problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Embodiment 1: Buffer system and pH screening experiment
[0117] In liquid pharmaceutical compositions, the buffer system and pH closely affect the stability of antibodies, and each antibody with unique physical and chemical properties has the most suitable buffer type and pH. This example aims to screen an optimal buffer system and pH so that the anti-BTLA antibody disclosed in the present invention has the best stability and is suitable for clinical application.
[0118] This example was performed with antibody hu18 at concentrations of about 10 mg / mL, 20 mg / mL and 40 mg / mL. The samples were ultrafiltered and concentrated using VIVAFlow200 to change the liquid. After the liquid was changed, the samples were in the corresponding prescription, and the samples were placed in a closed centrifuge tube for buffer screening. Acetate buffer, citrate buffer and histidine buffer were screened, with pH ranging from 5.0 to 6.5 (shown in Table 1). The samples were placed in an e...
Embodiment 2
[0129] Embodiment 2: Stabilizer screening experiment
[0130] In order to further explore the influence of different excipients on the stability of antibodies, we selected preparations of sodium chloride, mannitol, sorbitol, sucrose or trehalose or a combination thereof for comparative testing. That is, the above-mentioned different excipients or their combinations were added to 20 mM histidine buffer or citrate buffer containing about 20 mg / mL antibody hu18, and the specific prescription information is shown in Table 4. Each prescription preparation was placed under the condition of 40°C after subpackaging, and was taken out at week 0, week 2, and week 4 for analysis and detection. The content change of antibody hu18 monomer was detected by molecular exclusion high performance liquid chromatography (SEC-HPLC), and the content of the main charge peak of antibody hu18 was detected by weak cation high performance liquid chromatography (CEX-HPLC). The results are shown in Table ...
Embodiment 3
[0140] Embodiment 3: Surfactant screening experiment
[0141] The addition of surfactants to liquid formulations is often used to protect proteins such as antibodies from air / solution interface-induced stress, solution / surface-induced stress during storage to reduce antibody aggregation or minimize particle formation in formulations Reagent, which is beneficial to the stability of the physicochemical properties of the antibody. Different concentrations of polysorbate 20 were added to preparations containing 20 mM histidine buffer (the molar ratio of histidine to histidine hydrochloride was 1:1, pH 6.0) and 20 mg / ml of antibody hu18 Or polysorbate 80, after 4 weeks at 40°C, analyze and detect. The results are shown in Table 6.
[0142] Comprehensive analysis shows that the surfactant screening test (F17, F24, F25 and F26) results show that adding different concentrations of polysorbate 80 or polysorbate 20 has no effect on the monomer content of SEC-HPLC and the main peak con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| osmolarity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com