A preparation method and hydrogen storage material of graphene-supported nanosheet transition metal hydride
A technology of transition metals and nanosheets, applied in the direction of transition element hydrides, chemical instruments and methods, non-metallic elements, etc., can solve the problems of high kinetic energy barrier, difficulty in reversible hydrogen absorption, low hydrogen storage capacity, etc., to achieve It is beneficial to the improvement of capacity, hydrogen desorption kinetics and cycle performance, and the effect of small particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Preparation of graphene-supported nanosheet titanium hydride catalysts:
[0047] (1) Titanium tetrachloride (2 mmol), lithium hydride (8 mmol), graphene (20 mg) and tetrahydrofuran (50 ml) were weighed in an argon atmosphere glove box, added to a stainless steel reaction vessel, and sealed.
[0048] (2) Transfer the closed stainless steel reaction vessel to a magnetic heating stirrer, and keep it at 100°C for 2 hours or at 100°C for 4 hours or 200°C for 2 hours.
[0049] (3) Perform vacuum suction filtration on the product in step (2) in an inert atmosphere glove box to obtain a solid powder.
[0050] (4) heating the solid powder obtained in step (3) to 70° C. for 6 hours under dynamic vacuum to remove residual tetrahydrofuran, and finally obtain a graphene-supported nano-sheet titanium hydride catalyst.
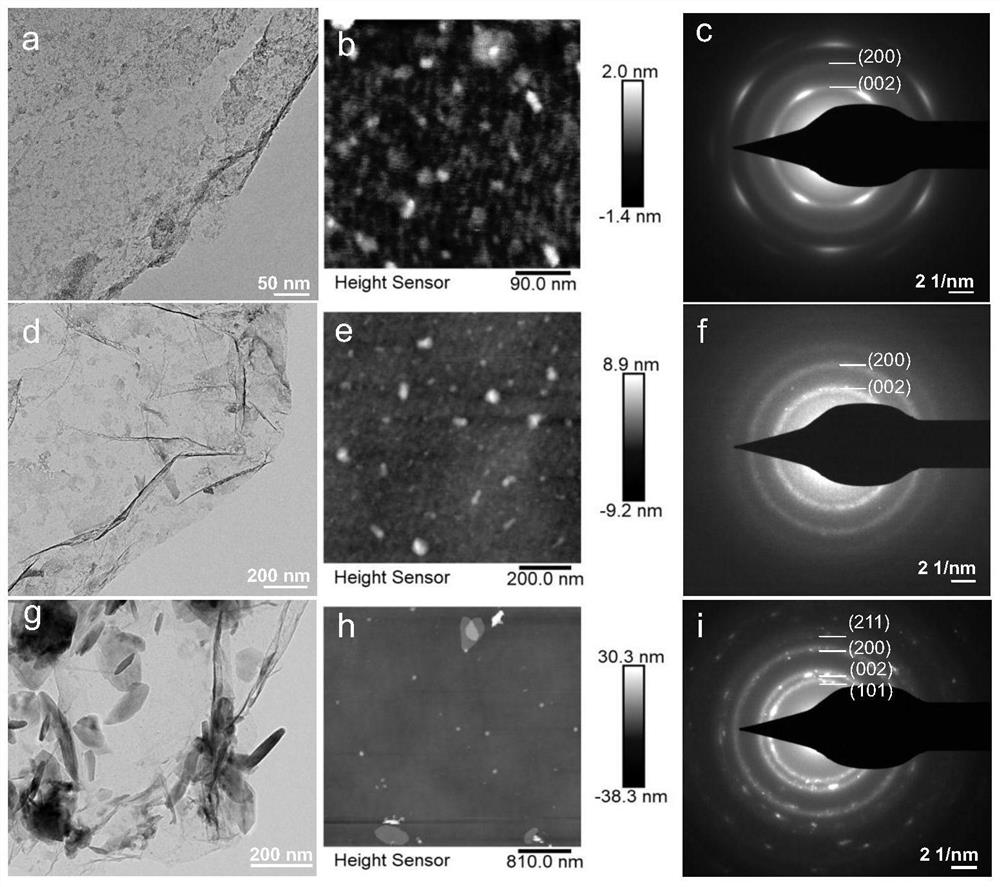
[0051] The samples prepared in the above process are: graphene-supported nano-sheet titanium hydride catalyst (NF-TiH 2 @G). figure 1 a is the transmission electro...
Embodiment 2
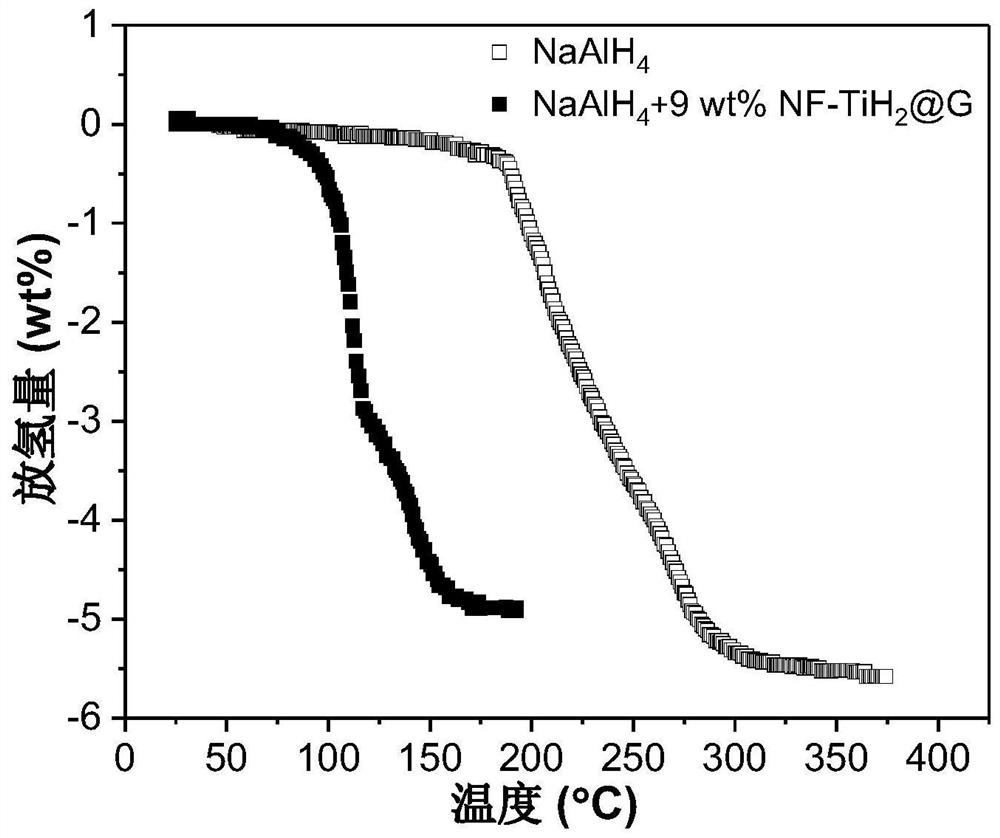
[0055] Add NF-TiH 2 @G's NaAlH 4 Preparation of hydrogen storage material: the NF-TiH of Example 1 (step 2 was kept at 100 °C for 2 h) 2 @G as catalyst with NaAlH 4 As a hydrogen storage material, NF-TiH was weighed separately in an argon atmosphere glove box 2 @G and NaAlH 4 , added to the ball mill jar, sealed, where NF-TiH 2 The mass fraction of @G in the mixture is 9 wt%. Transfer the ball mill jar to a ball mill, and perform ball milling. The ball milling speed is 500 rpm, the ball-to-material ratio is 120:1, and the ball milling time is 24 hours to obtain the hydrogen storage material NaAlH 4 +9wt%NF-TiH 2 @G. The hydrogen desorption kinetic performance of the hydrogen storage material was tested by the volume method, and the test conditions were under vacuum (the initial vacuum degree was 1×10 -3 Torr) was heated to 250°C at a heating rate of 2°C / min, the results are shown in figure 2 .
Embodiment 3
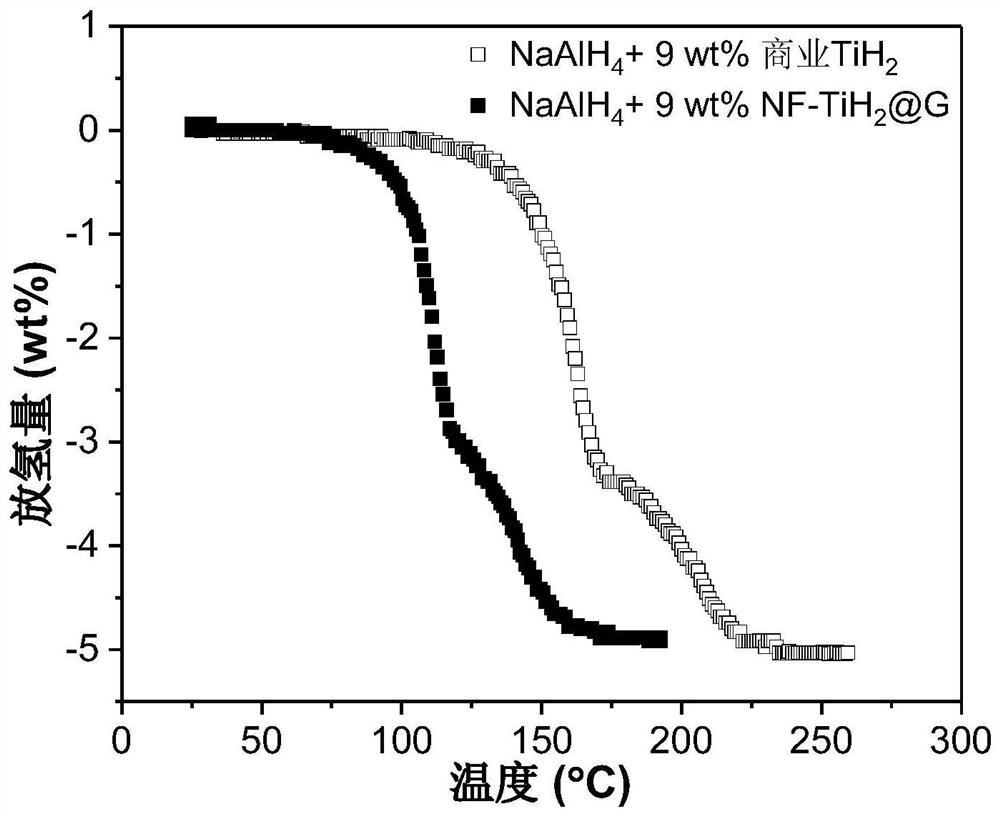
[0063] Add NF-TiH 2 @G's NaAlH 4 Preparation of hydrogen storage material: the NF-TiH of Example 1 (step 2 was kept at 100 °C for 2 h) 2 @G as catalyst with NaAlH 4 As a hydrogen storage material, NF-TiH was weighed separately in an argon atmosphere glove box 2 @G and NaAlH 4 , added to the ball mill jar, sealed, where NF-TiH 2The mass fraction of @G in the mixture is 9 wt%. Transfer the ball mill jar to a ball mill, and perform ball milling. The ball milling speed is 500 rpm, the ball-to-material ratio is 120:1, and the ball milling time is 24 hours to obtain the hydrogen storage material NaAlH 4 +9wt%NF-TiH 2 @G. The hydrogen desorption kinetic performance of the hydrogen storage material was tested by TPD (hydrogen release with temperature). The test conditions were heating to 250°C at a heating rate of 2°C / min under argon carrier gas. The results are shown in Figure 4 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com