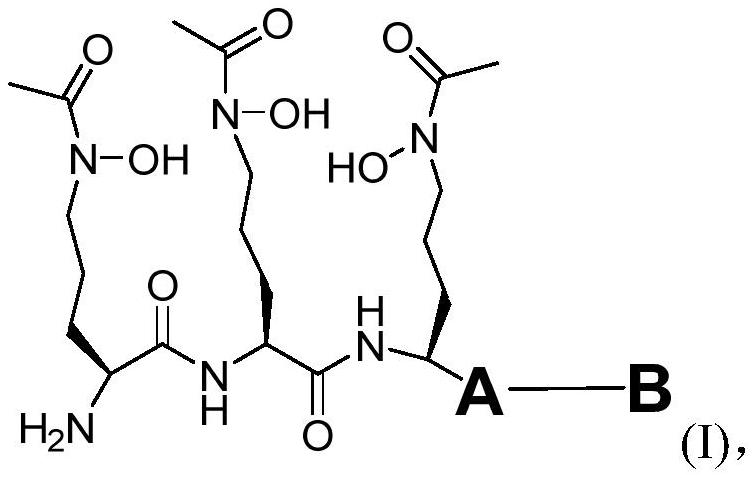
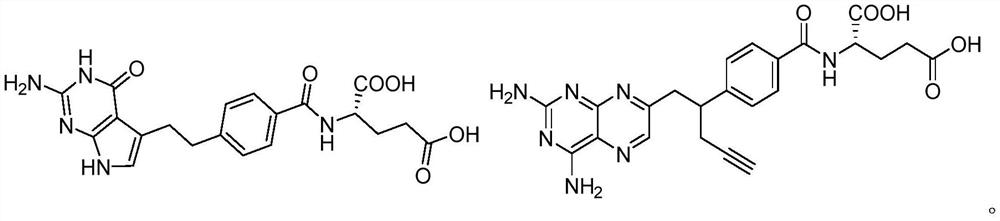
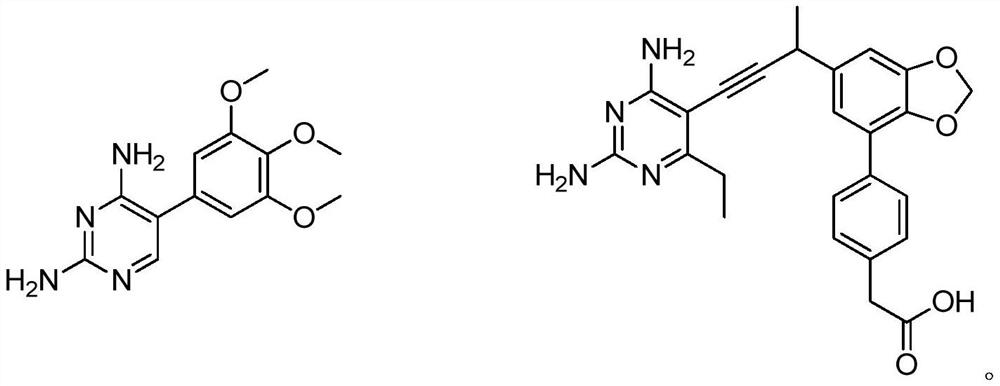
Iron carrier-dihydrofolate reductase inhibitor conjugate and application thereof
A reductase inhibitor, dihydrofolate technology, applied in the field of compounds, can solve problems such as high toxicity and the use of antibacterial reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] Synthesis of compound 3-4a: as shown in the scheme below, siderophore fragment 2-81 was condensed with compound 3-5 under HATU and DIPEA conditions to obtain compound 3-1a. Compound 3-1a was deprotected from Boc with trifluoroacetic acid, and then coupled with tert-butyl ester-protected methotrexate 3-8 to obtain compound 3-3a. The same tert-butyl ester protection was removed with trifluoroacetic acid, and then K 2 CO 3 The remaining Bz and Fmoc protecting groups were removed to obtain the target product 3-4a.
[0130]
[0131] 1) Preparation of compound 3-1a
[0132] Under nitrogen protection, compound 2-81 (300 mg, 0.281 mmol, 1.0 equiv), compound 3-5 (45 mg, 0.281 mmol, 1.0 equiv), HATU (160 mg, 0.421 mmol, 1.5 equiv) were dissolved in dry DMF ( 5 mL), placed in a low-temperature reactor at -15°C and stirred, and slowly added DIPEA (93 μL, 0.562 mmol, 2.0 equiv) dropwise. After reacting for 2 hours, the pH of the reaction solution was adjusted to neutral with ...
example 2-11
[0142] The synthesis of example 3-12 is the same as the synthesis route of example 1, and 3-4b, 3-4c, 3-4f, 3-4j, 3-4k, 3-4l, 3-4n, 3-4p, 3- 4r,3-4u.
Embodiment 12
[0144] Synthesis of compound 3-4e: as shown in the scheme below, siderophore fragment 2-81 was condensed with compound 3-5 under HATU and DIPEA conditions to obtain compound 3-1e. Compound 3-1e was deprotected from Boc with trifluoroacetic acid, and then coupled with Boc-protected serine 3-7 to obtain compound 3-2e. Similarly, the Boc protection was removed with trifluoroacetic acid, and then condensed with tert-butyl ester-protected methotrexate 3-8 to obtain compound 3-3e. Finally, use trifluoroacetic acid to remove the protection of tert-butyl ester, and then use K 2 CO 3 The remaining Bz and Fmoc protecting groups were removed to obtain the target product 3-4e.
[0145]
[0146] in:
[0147] 1) Preparation of compound 3-1a
[0148] Under nitrogen protection, compound 2-81 (300 mg, 0.281 mmol, 1.0 equiv), compound 3-5 (45 mg, 0.281 mmol, 1.0 equiv), HATU (160 mg, 0.421 mmol, 1.5 equiv) were dissolved in dry DMF ( 5 mL), placed in a low-temperature reactor at -15°C a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com