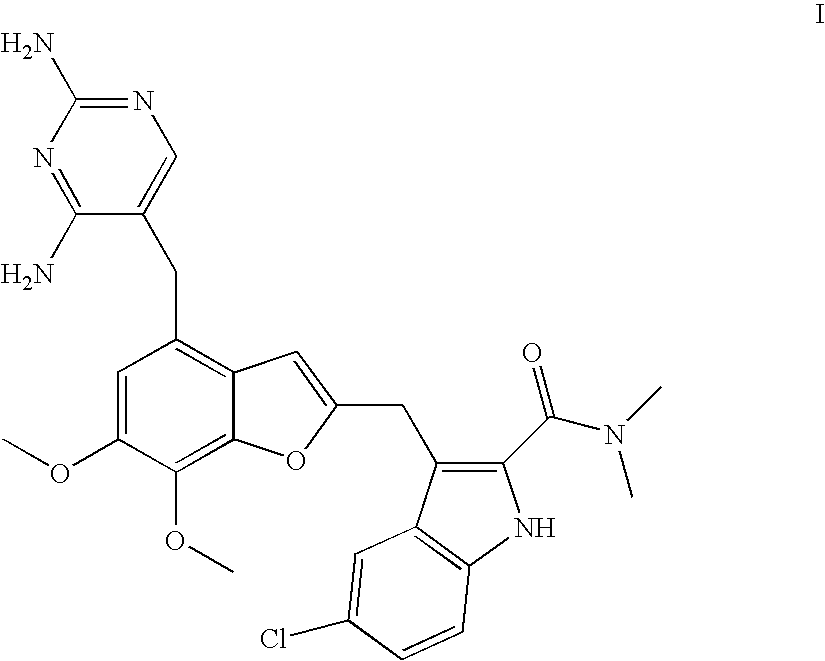
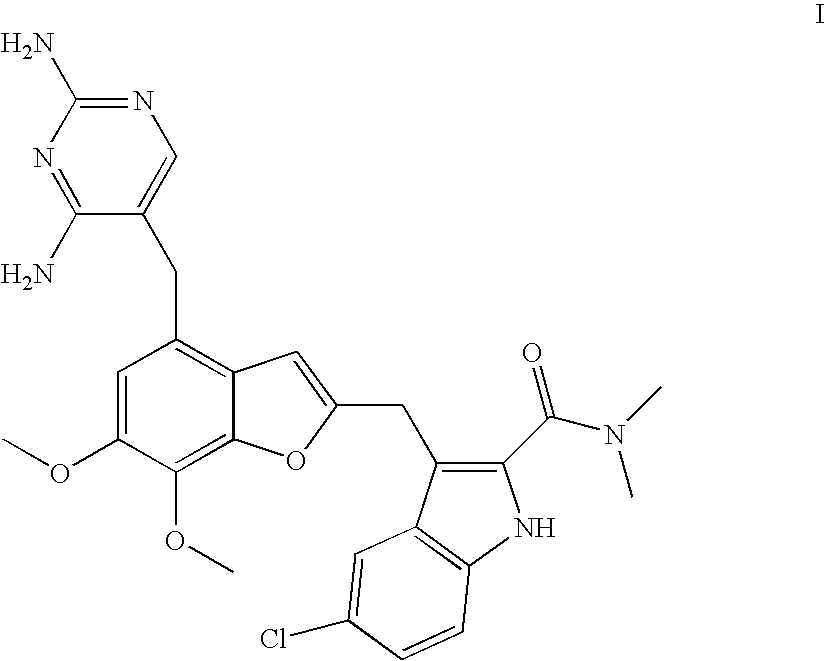
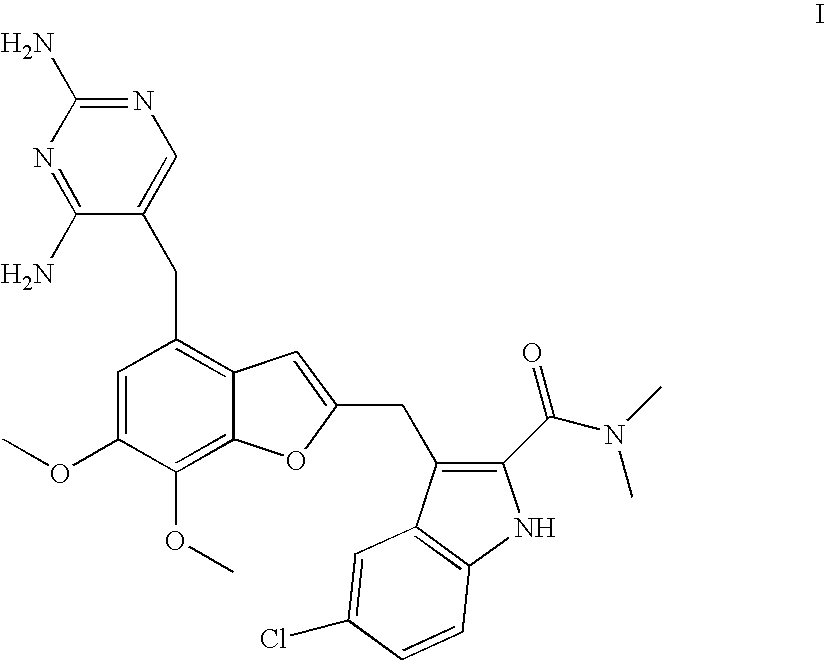
Patents
Literature
34 results about "Dihydrofolate reductase inhibitor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A dihydrofolate reductase inhibitor (DHFR inhibitor) is a molecule that inhibits the function of dihydrofolate reductase, and is a type of antifolate. Since folate is needed by rapidly dividing cells to make thymine, this effect may be used to therapeutic advantage. For example, methotrexate is used as cancer chemotherapy because it can prevent neoplastic cells from dividing. Bacteria also need DHFR to grow and multiply and hence inhibitors selective for bacterial vs. host DHFR have found application as antibacterial agents. An extensive review of the chemical space of small-molecules that inhibit DHFR is summarized in...
Heterocyclic and Cyclic Analogs of Propargyl-Linked Inhibitors of Dihydrofolate Reductase
ActiveUS20150225353A1BiocideOrganic active ingredientsDihydrofolate reductase inhibitorDihydrofolate reductase deficiency
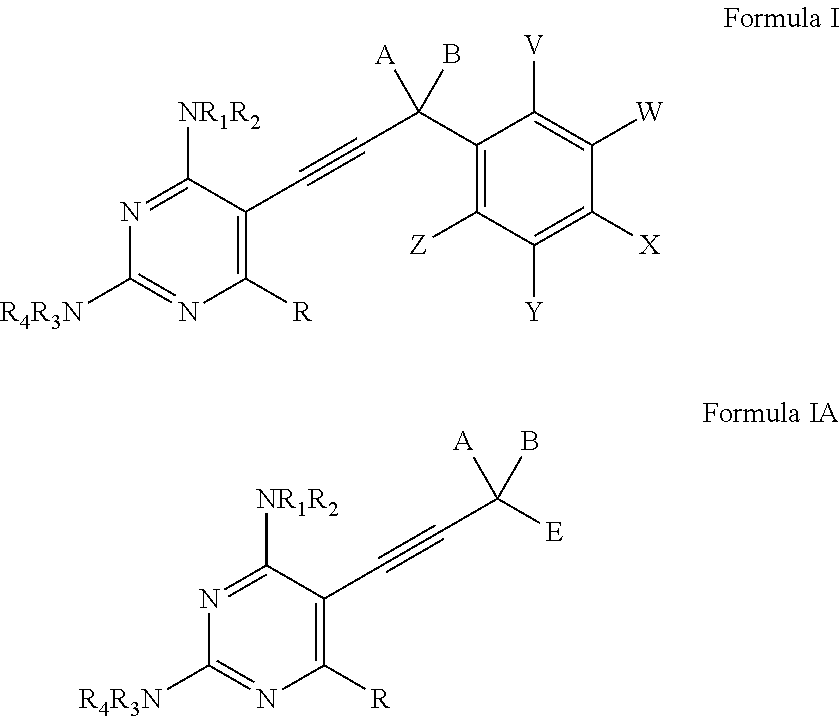
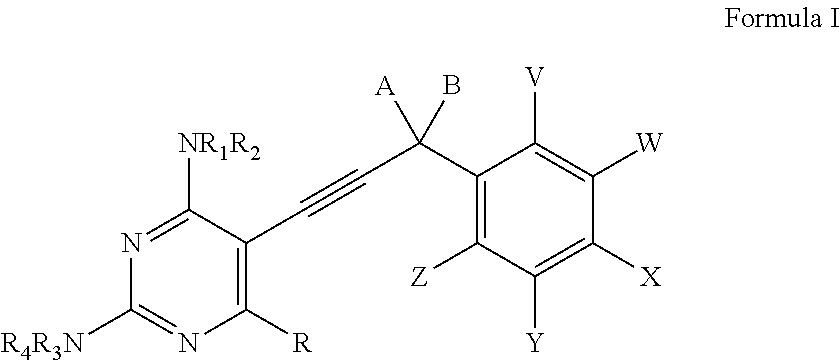
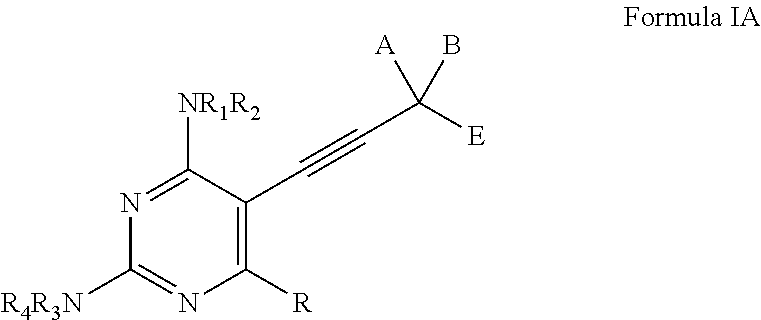
Compounds of Formula I and Formula IA are inhibitors of dihydrofolate reductase and are suitable for use in compositions and methods for dihydrofolate reductase inhibition or, more specifically, treatment of a fungal infection, a bacterial infection or a protozoal infection, and, in specific embodiments, treatment of a fungal infection caused by C. albicans or C. glabrata:wherein R, R1, R2, R3, R4, A, B, E, V, W, X, Y and Z are as defined herein.
Owner:PROMILIAD BIOPHARMA INC +1
DHFR enzyme inhibitors
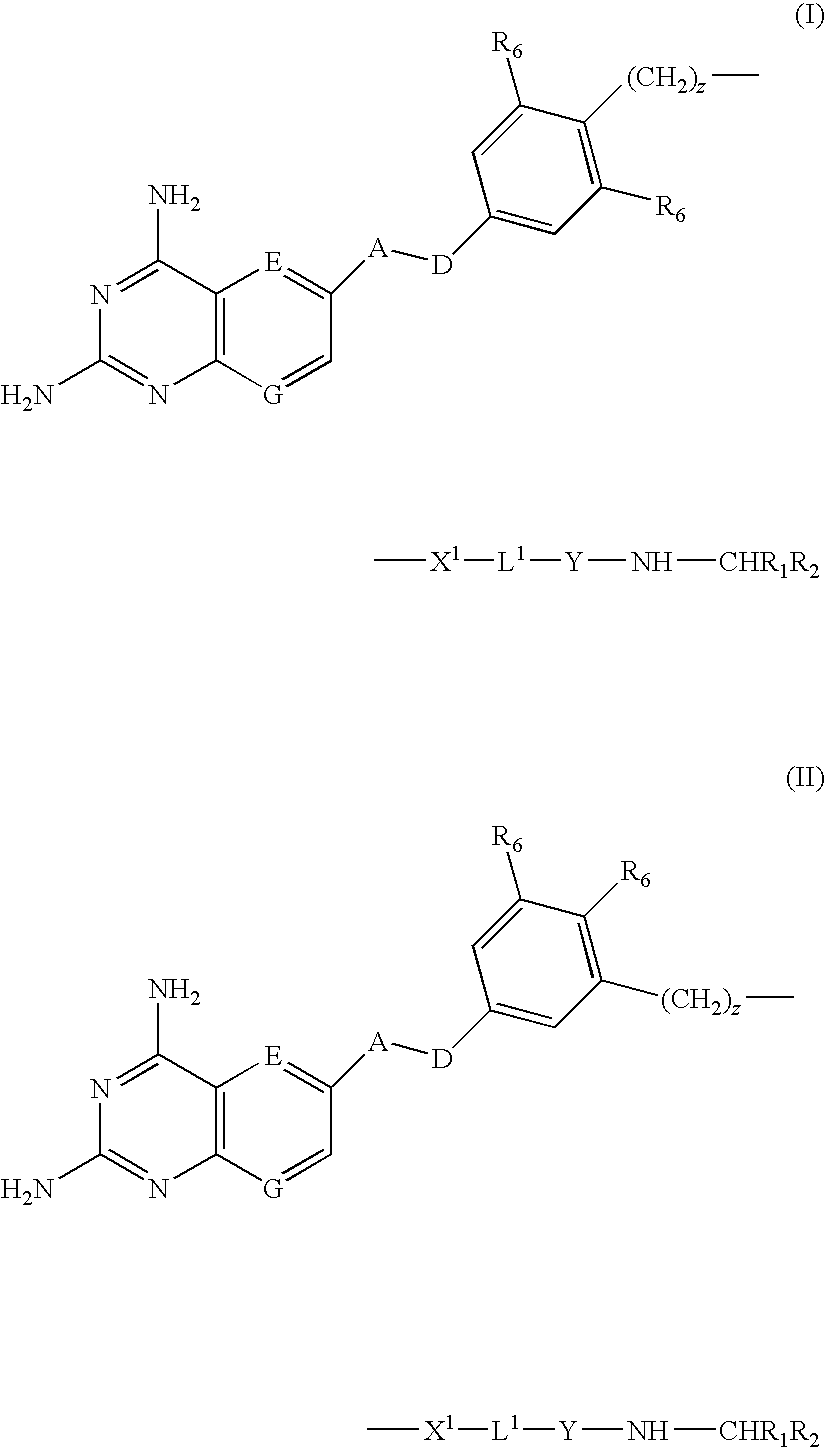
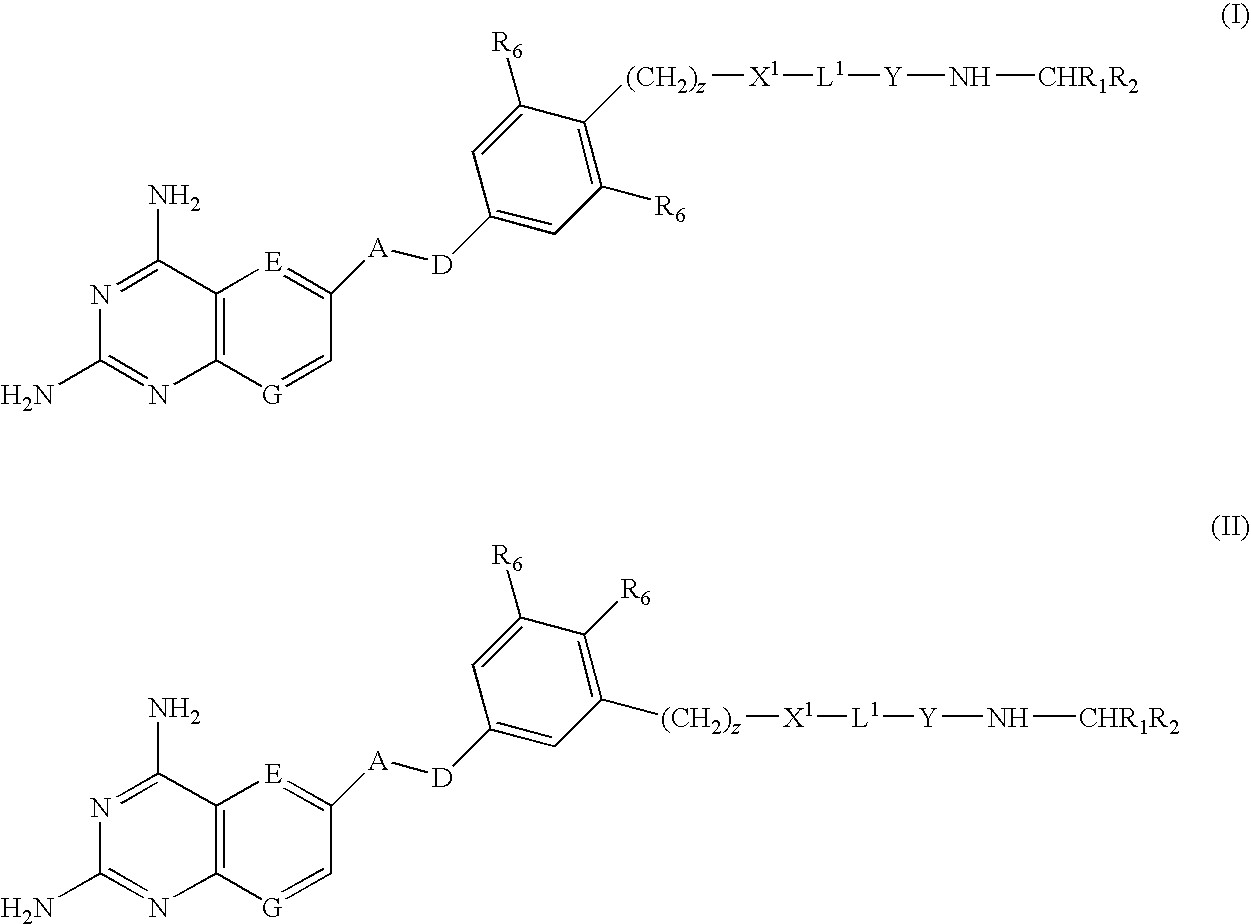
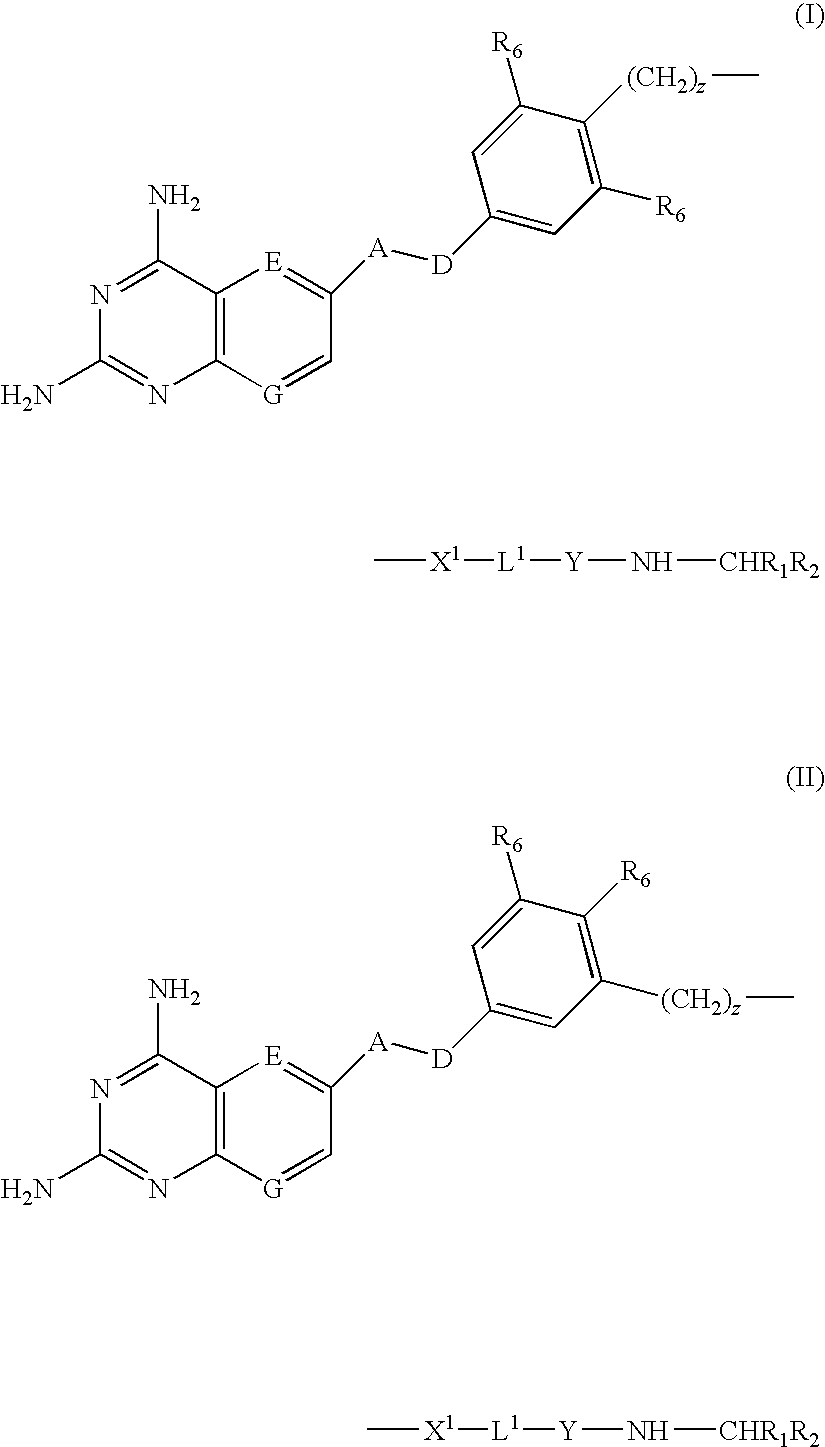
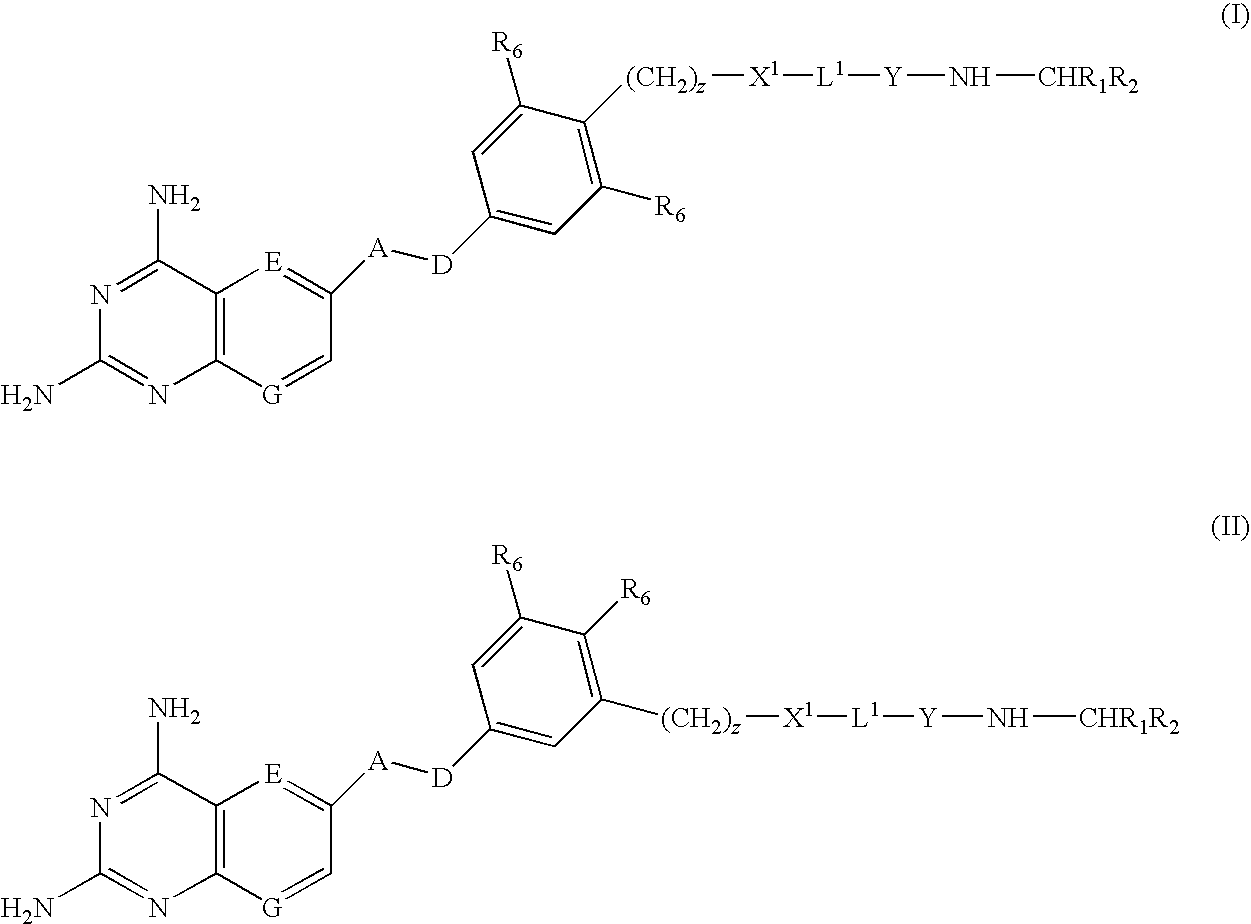
Compounds of formula (I) or (II) are dihydrofolate reductase inhibitors, useful for the treatment of, for example, cell proliferative diseases:wherein A and D are independently —CHR7— or —NR7—; E and G are independently ═CR7— or ═N—; each R6 independently represents hydrogen or —OR7; R7 is hydrogen or C1-C6 alkyl; R1 is a carboxylic acid group (—COOH), or an ester group which is hydrolysable by one or more intracellular carboxylesterase enzymes to a carboxylic acid group; R2 is the side chain of a natural or non-natural alpha amino acid which does not contain a carboxyl, or carboxyl ester group; Y is a bond, —C(═O)—, —S(═O)2—, —C(═O)NR3—, —C(═S)—NR3, —C(═NH)NR3 or —S(═O)2NR3— wherein R3 is hydrogen or optionally substituted C1-C6 alkyl; L1 is a divalent radical of formula -(Alk1)m(Q)n(Alk2)p- wherein m, n and p are independently 0 or 1, and Q, Alk1 and Alk2 are as defined in the claims; X1 represents a bond; —C(═O); or —S(═O)2—; —NR4C(═O)—, —C(═O)NR4—, —NR4C(═O)NR5—, —NR4S(═O)2—, or —S(═O)2NR4— wherein R4 and R5 are independently hydrogen or optionally substituted C1-C6 alkyl; and z is 0 or 1.
Owner:CHROMA THERAPEUTICS
Inhibitors of dihydrofolate reductase with antibacterial antiprotozoal, antifungal and anticancer properties
ActiveUS8426432B2Increase capacityStraightforward to synthesizeAntibacterial agentsBiocideProtozoaBacteroides
Owner:UNIV OF CONNECTICUT
Inhibitors of Dihydrofolate Reductase With Antibacterial Antiprotozoal, Antifungal and Anticancer Properties
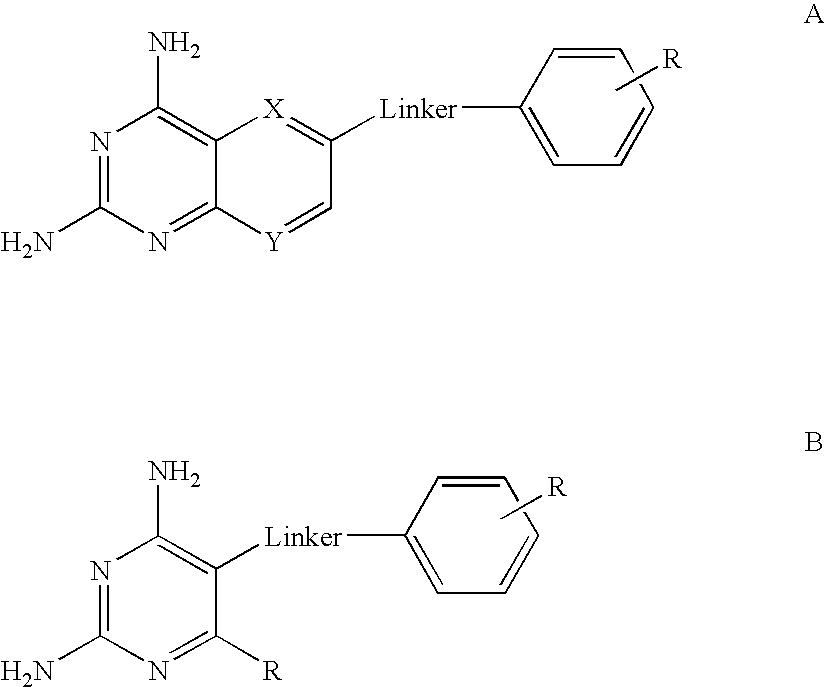
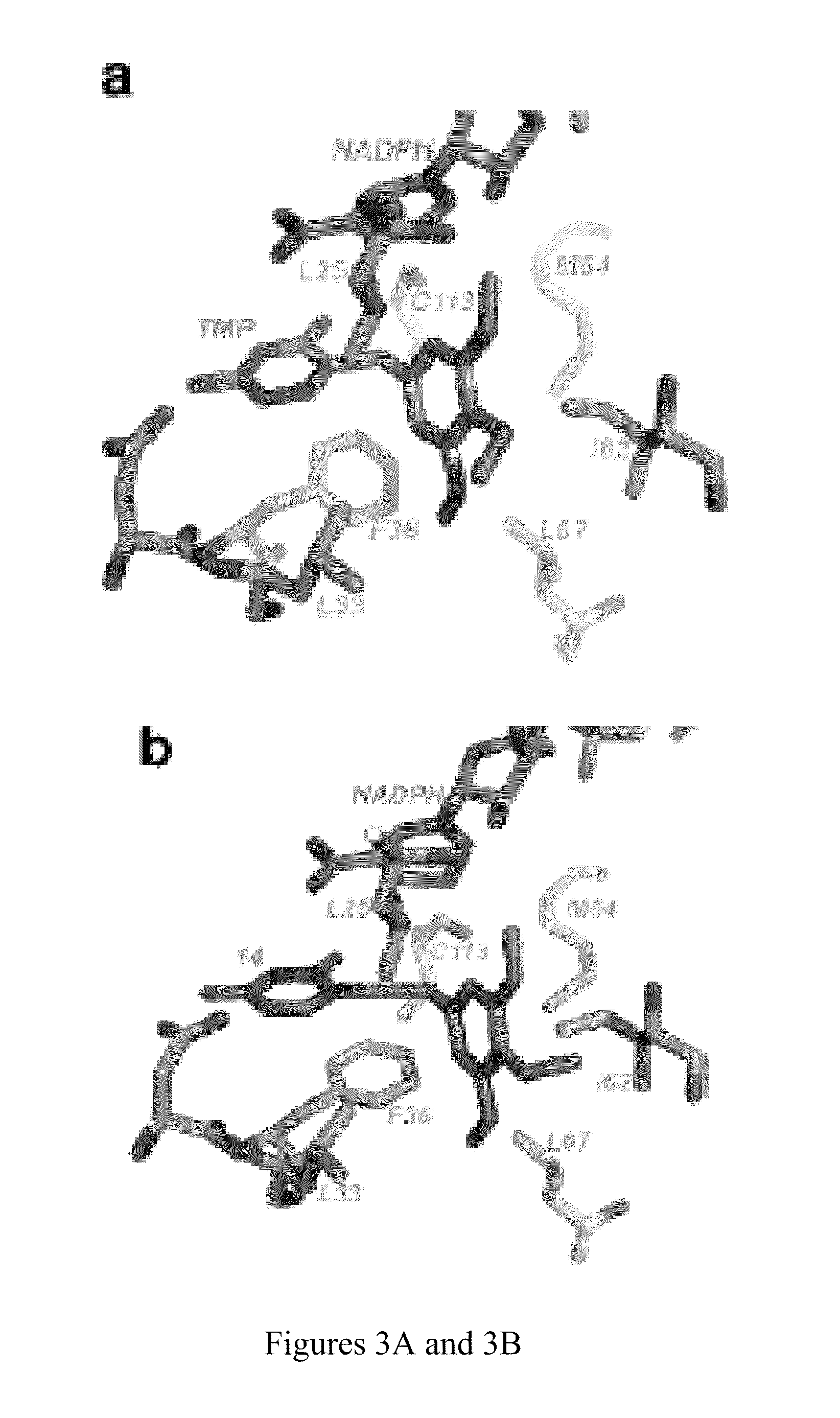
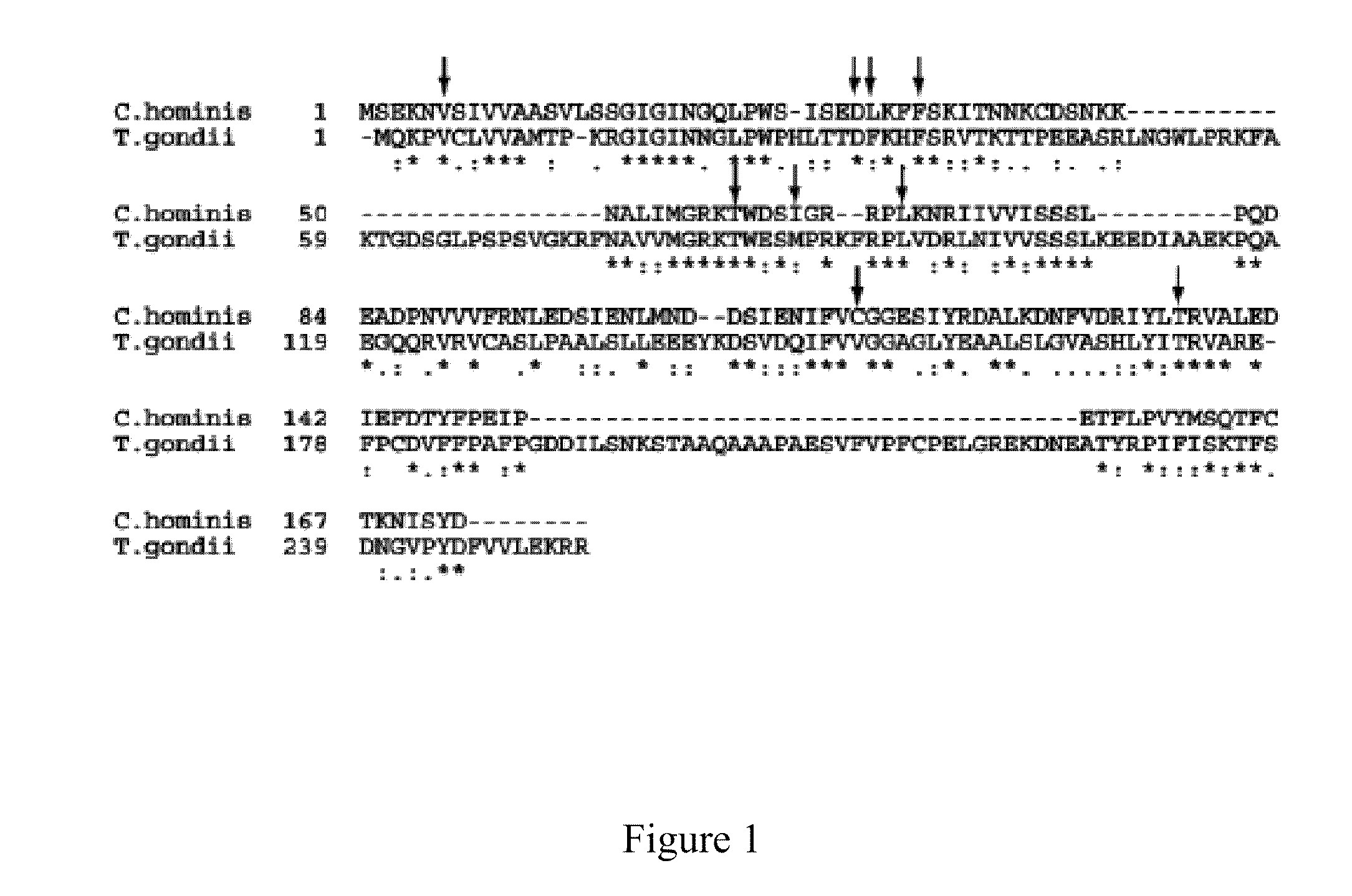
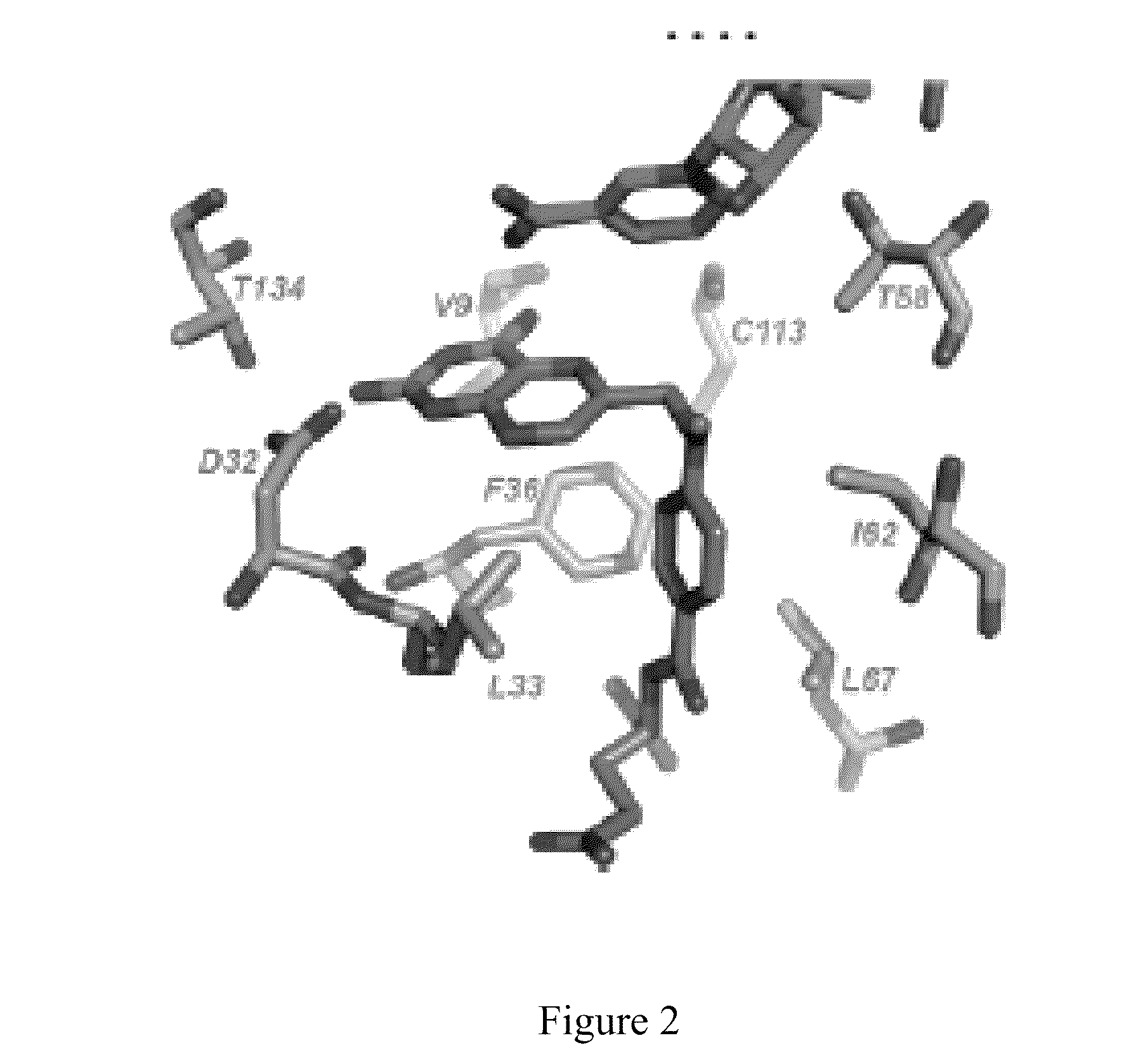
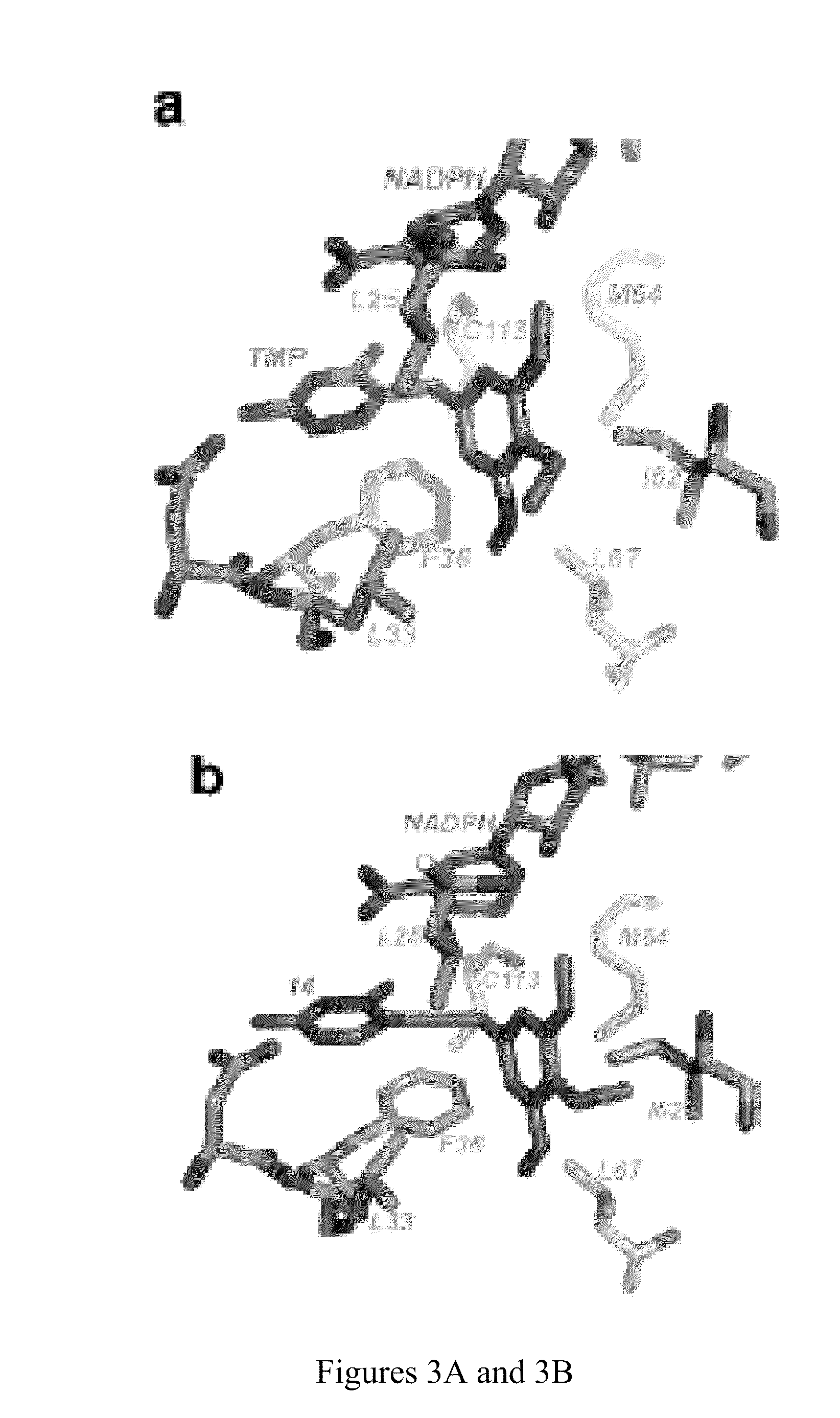
The compositions and methods described herein discloses the design, synthesis and testing of compounds that act as inhibitors of DHFR. The basic scaffold of these inhibitors includes a 2,4-diaminopyrimidine ring with a propargyl linker to another substituted aryl, bicyclo or heteroaryl ring. These DHFR inhibitors are potent and selective for many different pathogenic organisms, including the DHFR enzyme from bacteria such as Bacillus anthracis and methicillin-resistant Staphylococcus aureus, fungi such as Candida glabrata, Candida albicans and Cryptococcus neoformans and protozoa such as Cryptosporidium hominis and Toxoplasma gondii. These compounds and other similar compounds are also potent against the mammalian enzyme and may be useful as anti-cancer therapeutics.
Owner:UNIV OF CONNECTICUT
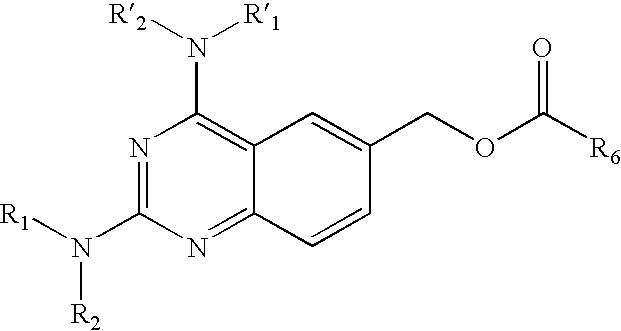
Diaminoquinazoline esters for use as dihydrofolate reductase inhibitors
Compounds of the formula I: wherein R1, R2, R1' and R2' are independently hydrogen or a group releasing the free amine in vivo, R6 is a substituted phenyl or an optionally substituted, bicyclic or tricyclic aryl ring system or an optionally substituted mono, bi- or tricyclic heteroaryl ring system having utility as DHFR inhibitors with favourable pharmacokinetic properties.
Owner:MELACURE THERAPEUTICS AB
Dihydrofolate reductase inhibitors
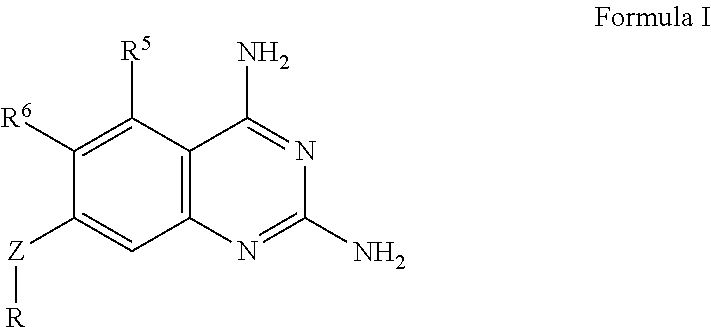
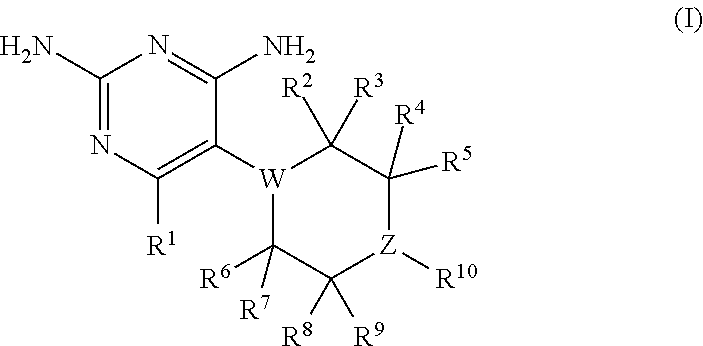
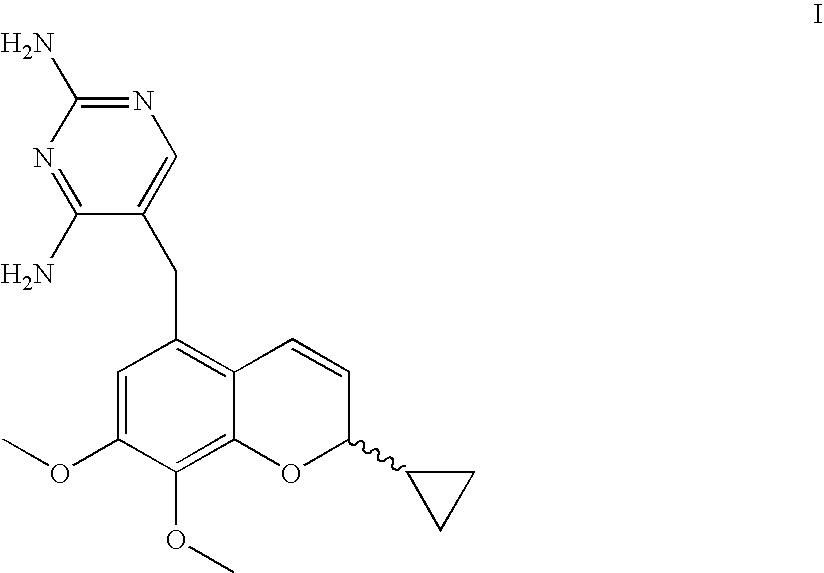
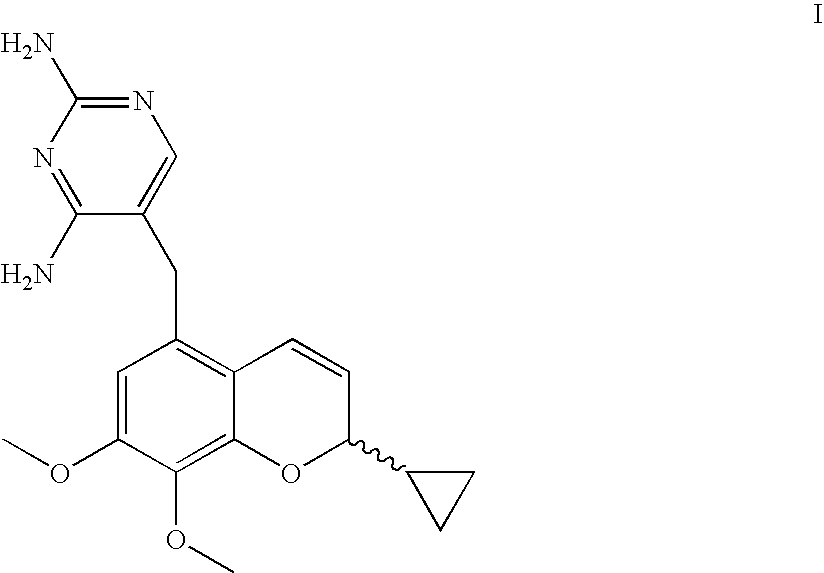
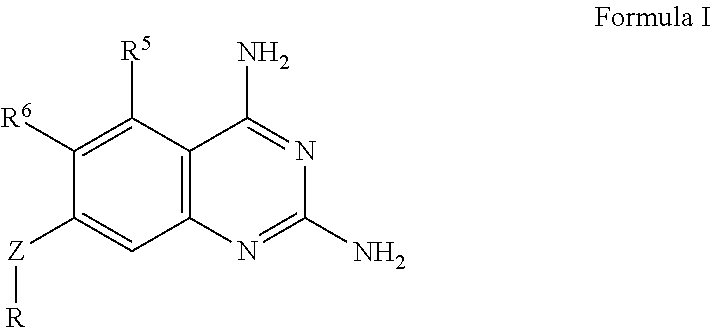
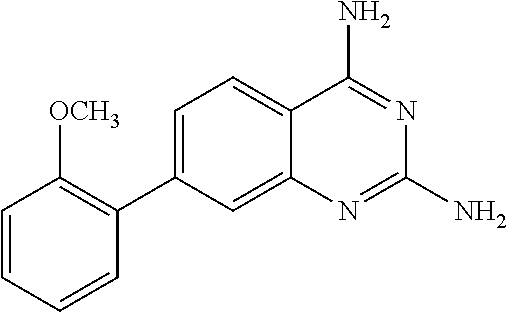
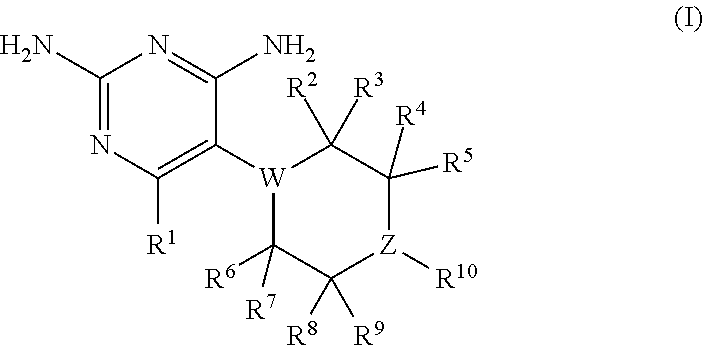
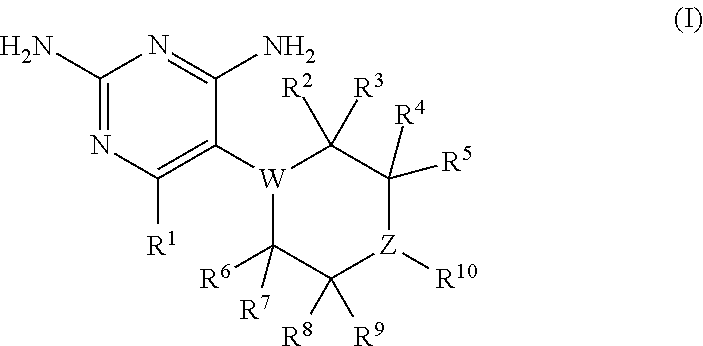
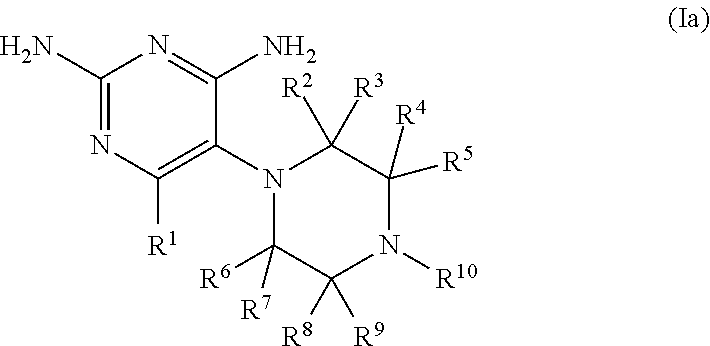
The present disclosure provides compounds of Formula I:or a pharmaceutically acceptable salt thereof, wherein R5, R6 and Z are as described herein. The disclosure also provides pharmaceutical compositions thereof; and methods for inhibiting DHFR activity; and methods for treating cell proliferative diseases, autoimmune disease, inflammatory disease or bacterial, fungal or parasitic infection by administering a compound of Formula I.
Owner:MERCK SHARP & DOHME LLC
DHFR Enzyme Inhibitors
InactiveUS20090118311A1Facilitates penetration of agentHigh potencyOrganic active ingredientsBiocideSide chainCarboxylic acid
Compounds of formula (I) or (II) are dihydrofolate reductase inhibitors, useful for the treatment of, for example, cell proliferative diseases:wherein A and D are independently —CHR7— or —NR7—; E and G are independently ═CR7— or ═N—; each R6 independently represents hydrogen or —OR7; R7 is hydrogen or C1-C6 alkyl; R1 is a carboxylic acid group (—COOH), or an ester group which is hydrolysable by one or more intracellular carboxylesterase enzymes to a carboxylic acid group; R2 is the side chain of a natural or non-natural alpha amino acid which does not contain a carboxyl, or carboxyl ester group; Y is a bond, —C(═O)—, —S(═O)2—, —C(═O)NR3—, —C(═S)—NR3, —C(═NH)NR3 or —S(═O)2NR3— wherein R3 is hydrogen or optionally substituted C1-C6 alkyl; L1 is a divalent radical of formula -(Alk1)m(Q)n(Alk2)p- wherein m, n and p are independently 0 or 1, and Q, Alk1 and Alk2 are as defined in the claims; X1 represents a bond; —C(═O); or —S(═O)2—; —NR4C(═O)—, —C(═O)NR4—, —NR4C(═O)NR5—, —NR4S(═O)2—, or —S(═O)2NR4— wherein R4 and R5 are independently hydrogen or optionally substituted C1-C6 alkyl; and z is 0 or 1.
Owner:CHROMA THERAPEUTICS
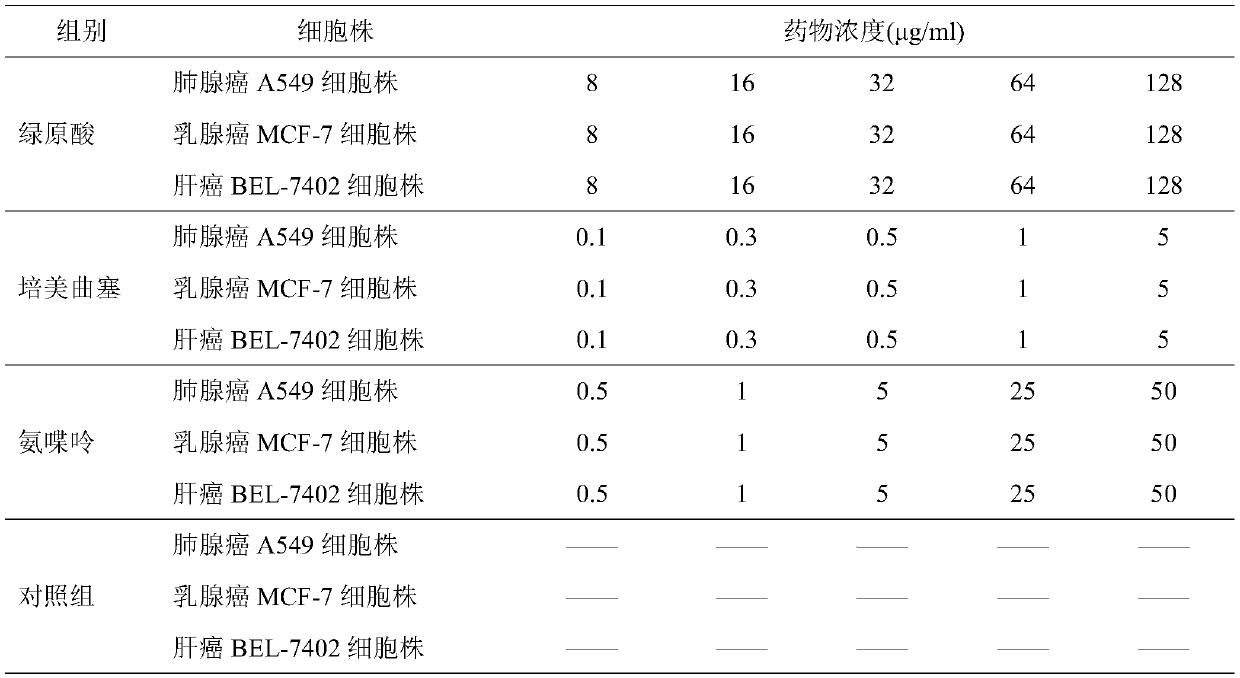
Combined medicine having efficacy of antineoplastic drugs
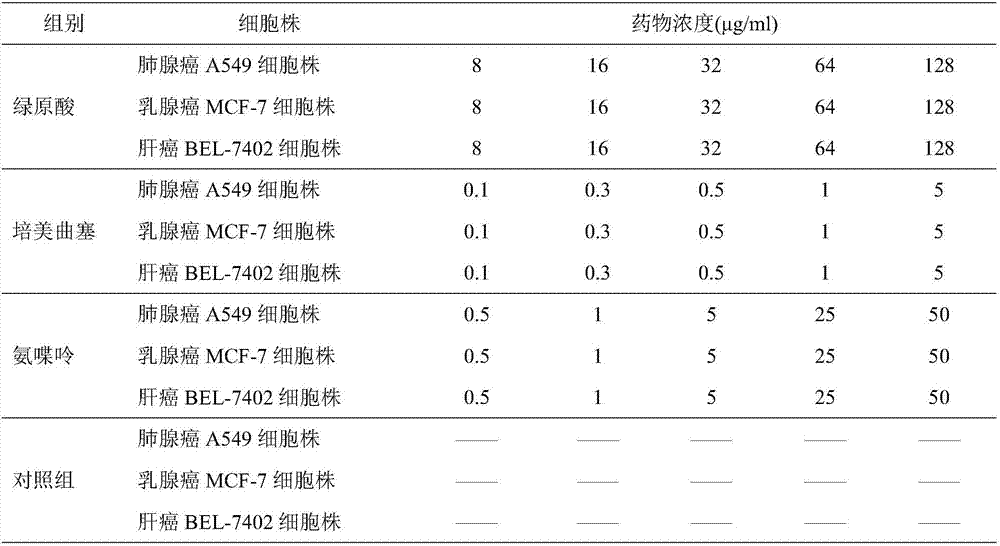
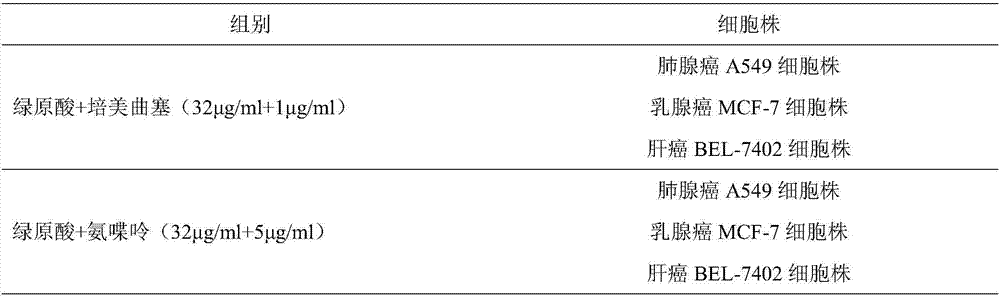
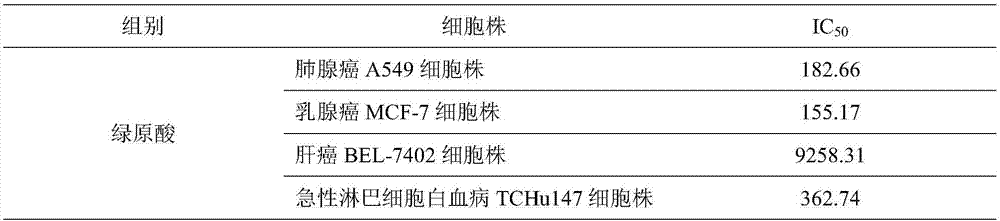
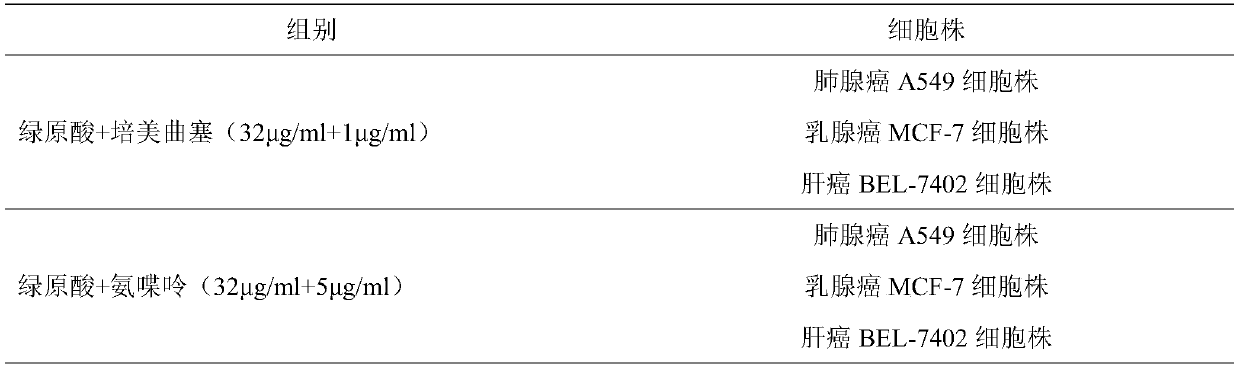
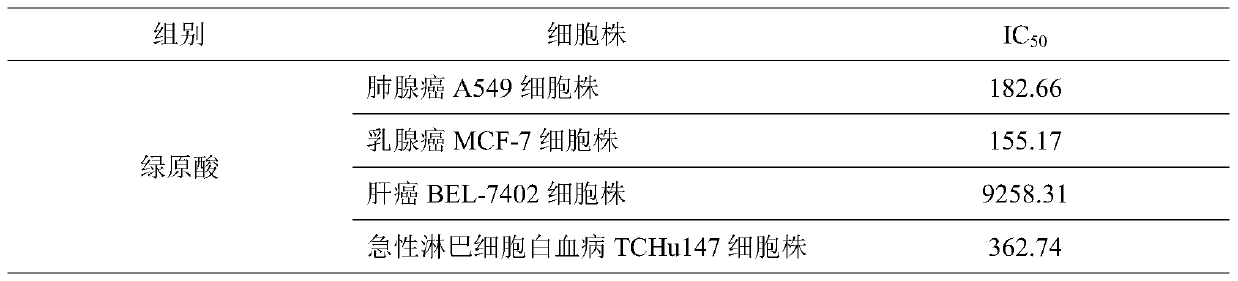
ActiveCN107496399AMitigation functionLose weightOrganic active ingredientsAntineoplastic agentsChlorogenic acidSide effect
The invention provides a combined medicine having efficacy of antineoplastic drugs. The combined medicine consists of chlorogenic acid and a dihydrofolate reductase inhibitor of unit preparations in same or different specifications, and a pharmaceutically acceptable carrier, wherein the chlorogenic acid and the dihydrofolate reductase inhibitor are applied in a simultaneous or separated mode. According to the combined medicine, a synergistic effect can be developed when the chlorogenic acid and the DHFR (dihydrofolate reductase) inhibitor are used in a combined mode, and hematopoietic function injury and weight loss caused by the DHFR inhibitor can be relieved; toxic and side effects of the DHFR inhibitor can be effectively reduced; and the medicine, which combines the chlorogenic acid and the DHFR inhibitor is excellent in curative effect, low in toxicity and good in prospect of clinical application.
Owner:SICHUAN JIUZHANG BIO TECH CO LTD
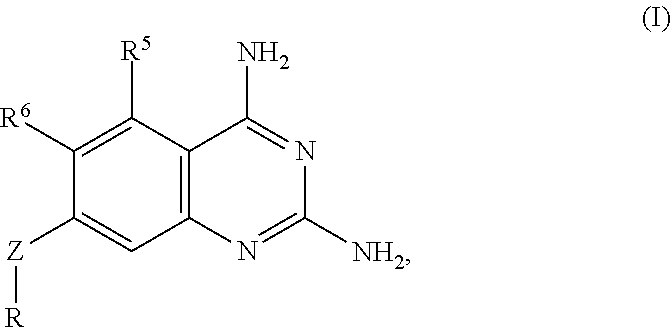
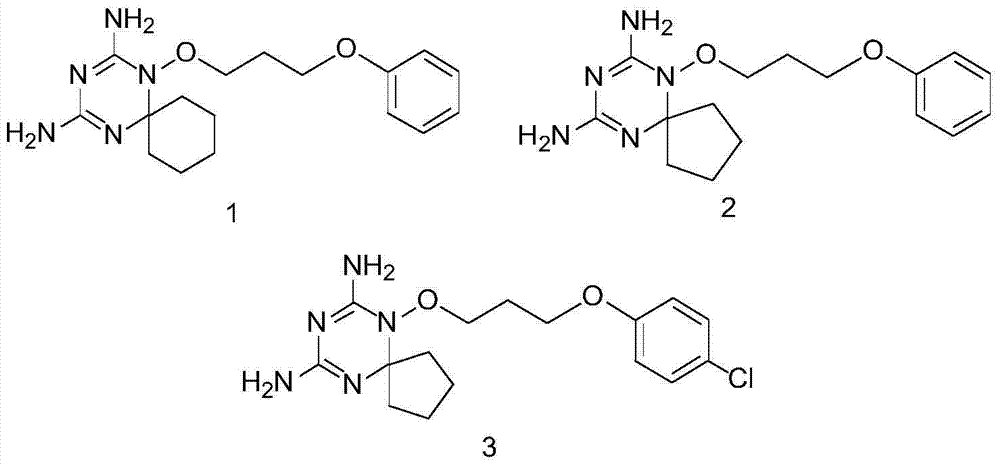
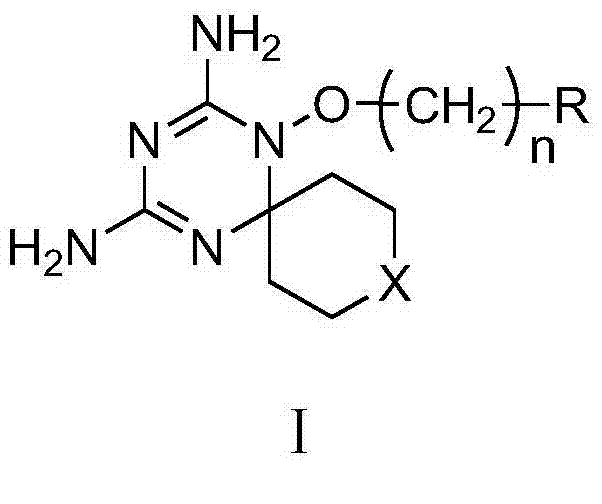
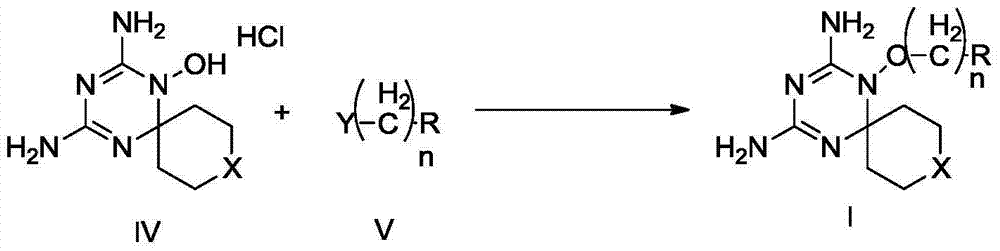
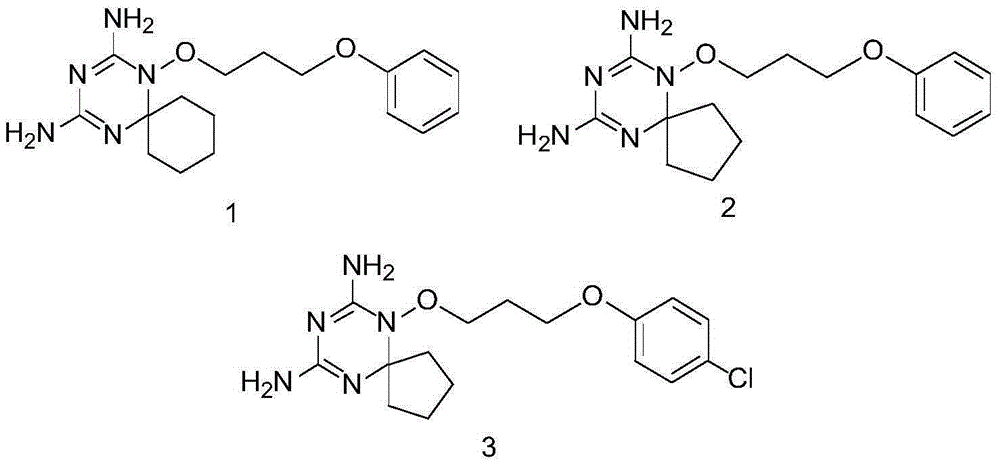
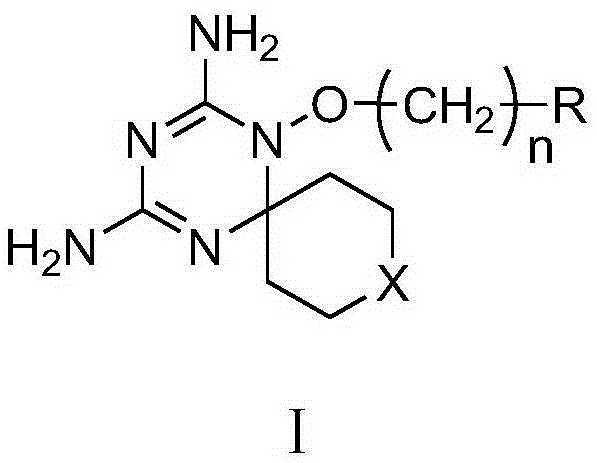
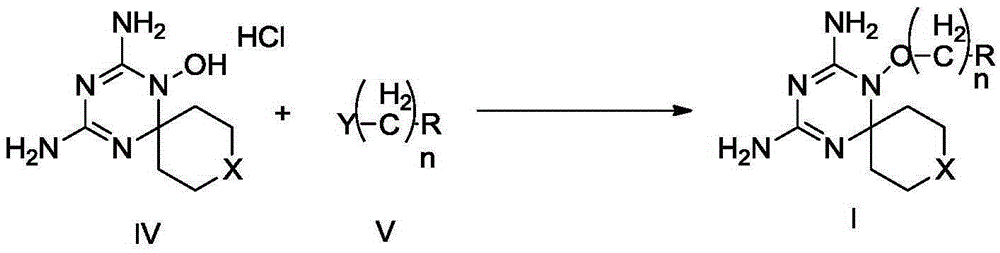
Derivatives and salts of diamino dihydrotriazine, and preparation method, composition and application thereof
ActiveCN103664972AStrong inhibitory activityBroaden research ideasAntibacterial agentsOrganic chemistryInfection diseaseDihydrofolate reductase inhibitor
The invention discloses derivatives and salts of damino dihydrotriazine, and a preparation method, a composition and application thereof. According to the invention, the preparation method of the damino dihydrotriazine derivative and the damino dihydrotriazine salt can be realized by adopting a method I or a method II, wherein the method I includes the step of obtaining a general formula I compound prepared through the reaction between a general formula IV compound and a general formula V compound, while the method II includes the step of mixing a general formula VIII compound with a general formula II compound under an acidic condition, and obtaining the compound shown in the general formula I through a cyclization reaction of the mixture. The invention also provides application of derivatives and salts of the damino dihydrotriazine in preparation of human dihydrofolate reductase inhibitors, preventing and curing drugs for tumor or bacterial infection diseases. The invention further provides a drug composition, which comprises an effective amount of the derivatives and / or salts of the damino dihydrotriazine, as well as pharmaceutically acceptable carriers. According to the invention, spiro heterocyclic ring derivatives of the damino dihydrotriazine have an excellent inhibitory activity on human dihydrofolate reductase, tumor cells and bacteria.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Heterocyclic analogs of propargyl-linked inhibitors of dihydrofolate reductase
ActiveUS20120196859A1Increase capacityStraightforward to synthesizeAntibacterial agentsBiocideBacteroidesProtozoa
The compositions and methods described herein disclose the design, synthesis and testing of compounds that act as inhibitors of DHFR. The basic scaffold of these inhibitors includes a 2,4-diaminopyrimidine ring with a propargyl linker to another substituted aryl, bicyclo or heteroaryl ring. These DHFR inhibitors are potent and selective for many different pathogenic organisms, including the DHFR enzyme from bacteria such as Bacillus anthracis and methicillin-resistant Staphylococcus aureus, fungi such as Candida glabrata, Candida albicans and Cryptococcus neoformans and protozoa such as Cryptosporidium hominis and Toxoplasma gondii. These compounds and other similar compounds are also potent against the mammalian enzyme and may be useful as anti-cancer therapeutics.
Owner:UNIV OF CONNECTICUT
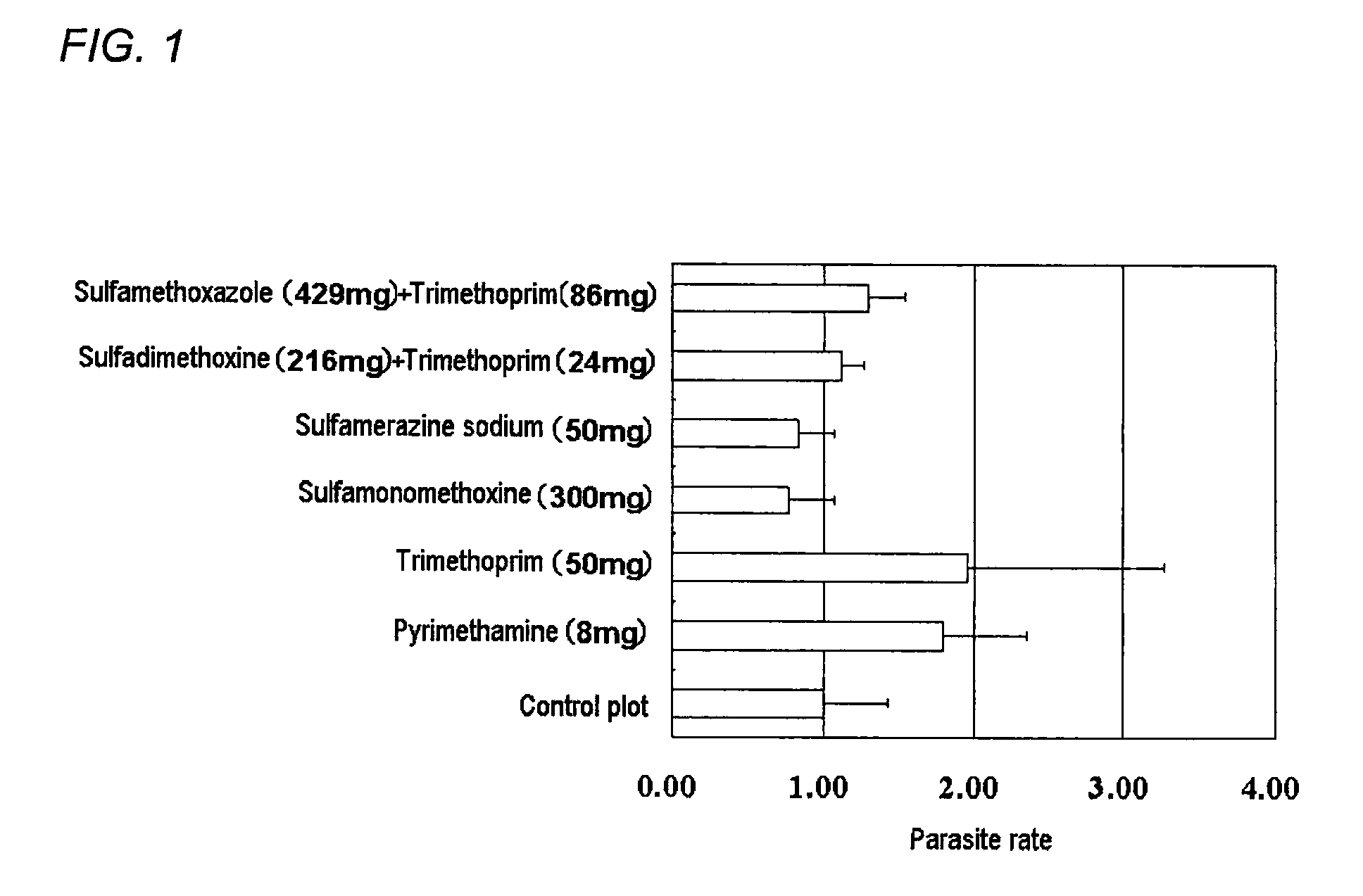
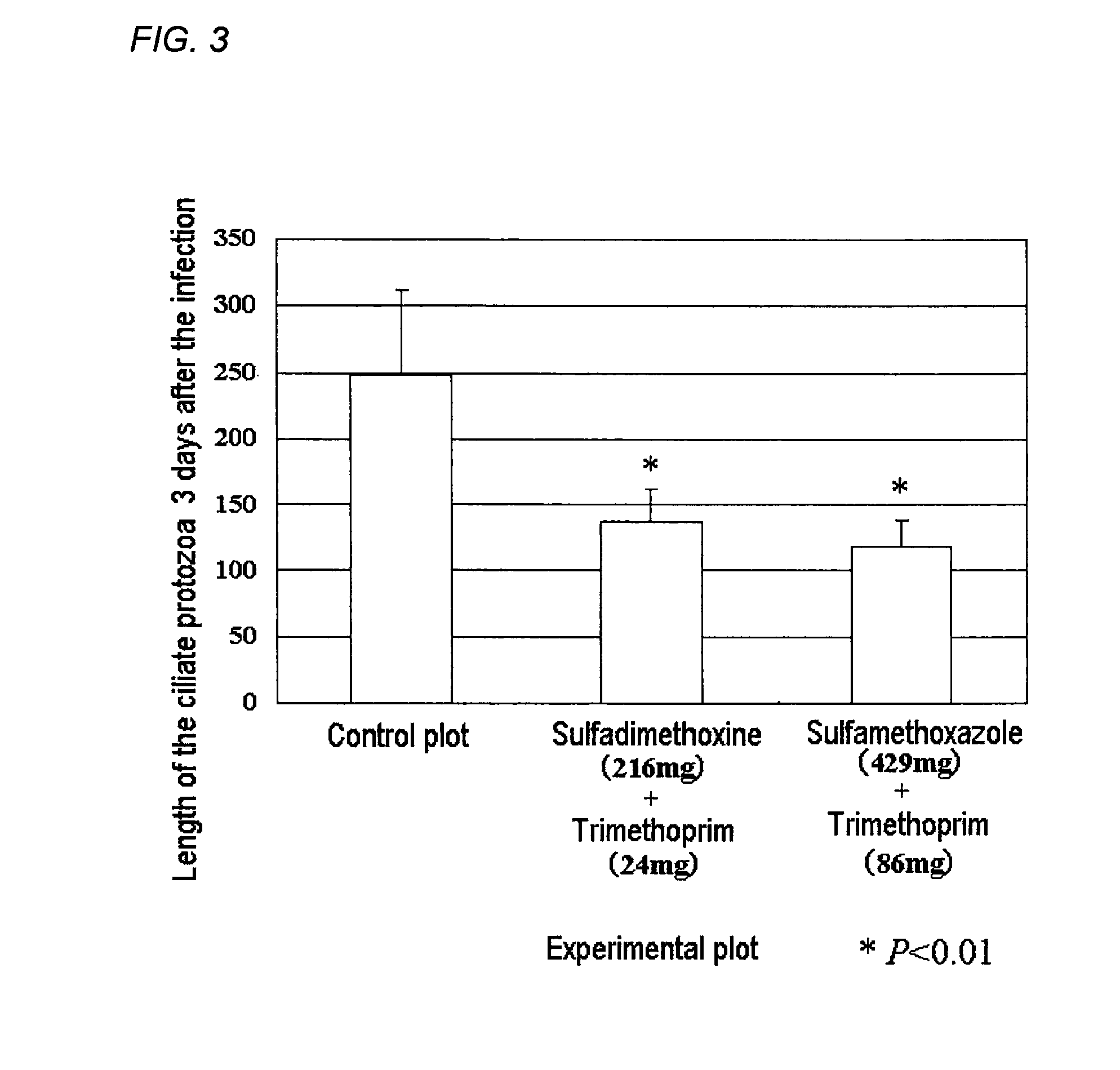
Method for control of fish parasites
InactiveCN102413841AReduce the burden onInhibition of resistant bacteriaOrganic chemistryPharmaceutical delivery mechanismFolic acid antagonistOral medication
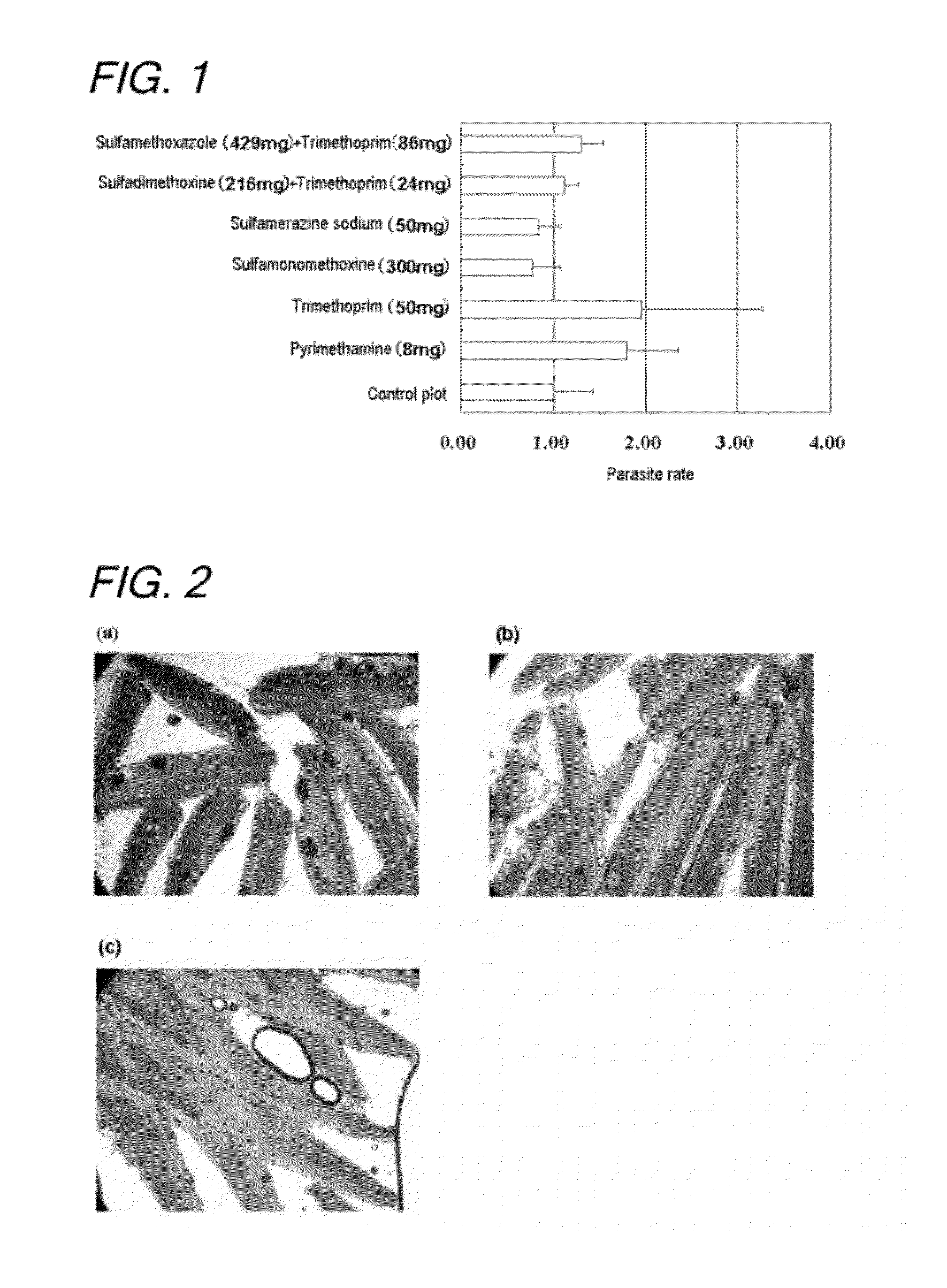
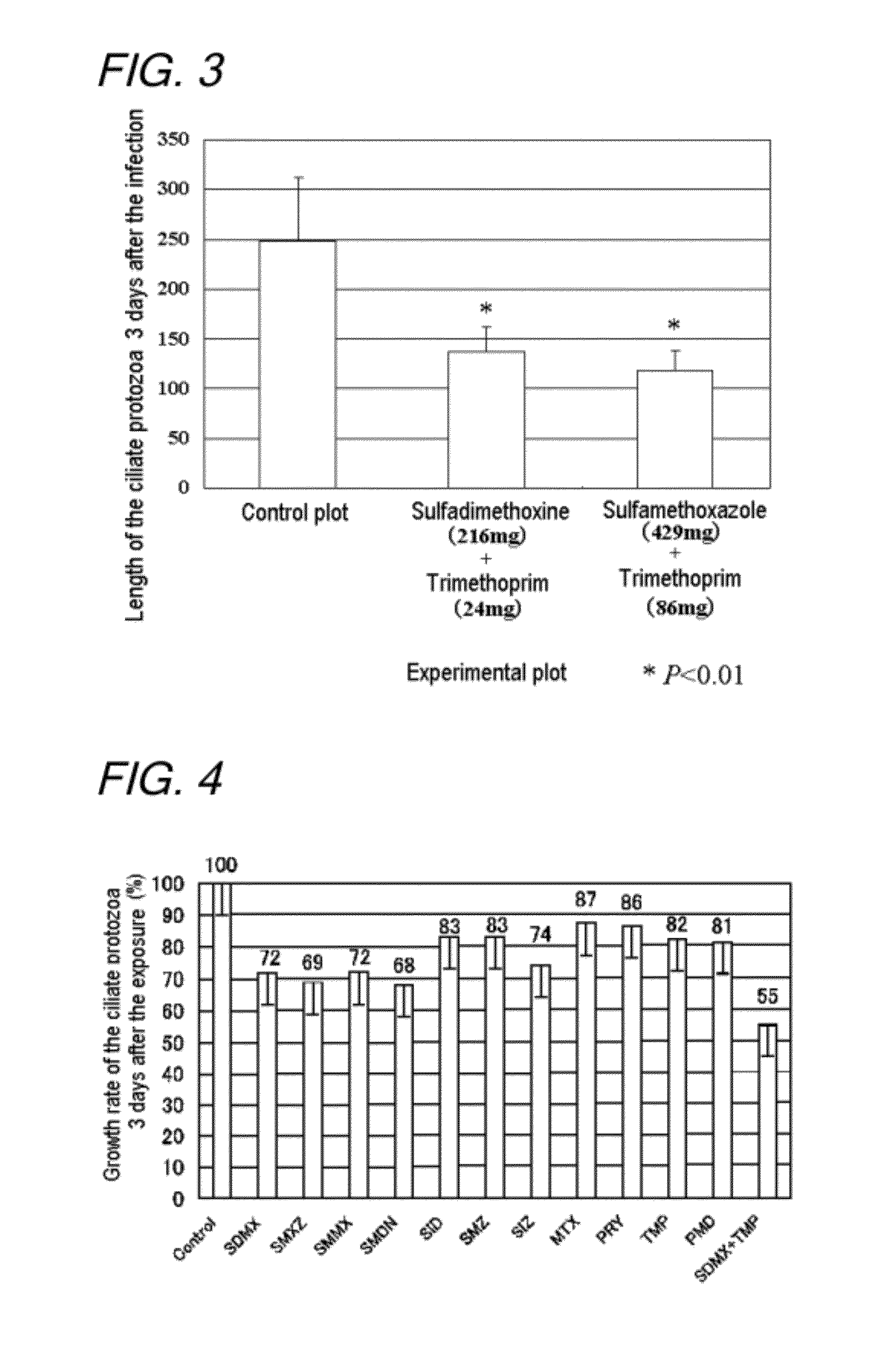
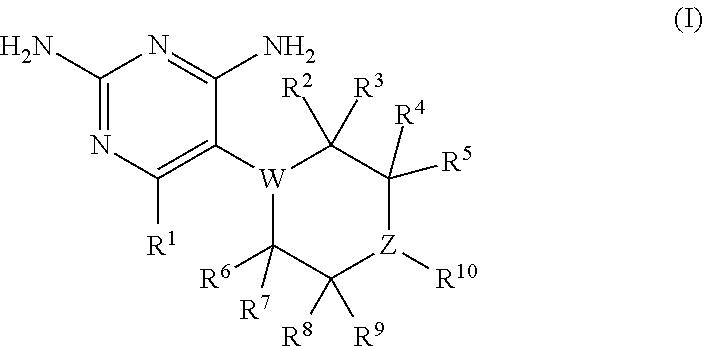
Disclosed is a method for controlling fish parasites, which comprises continuously administering a folic acid synthesis inhibitor and / or a folic acid activation inhibitor to a fish at a dose of 1 to 50 mg / kg fish body weight per day for one to two weeks. Preferably, a combination of a folic acid synthesis inhibitor and a folic acid activation inhibitor is used. As the folic acid synthesis inhibitor, a sulfa drug is preferred. As the folic acid activation inhibitor, a dihydrofolate reductase inhibitor, a folic acid antagonist, or the like can be used. The parasite control agent can control fish parasites through oral administration. The parasite control agent is particularly effective on ciliate fish parasites.
Owner:NIPPON SUISAN KAISHA LTD
Novel Processing for the Preparation of a Benzofuran
InactiveUS20080161561A1Antibacterial agentsOrganic chemistryDihydrofolate reductase inhibitorBenzofuran
The present invention relates to a novel process for the preparation of the compound of formula I, a dihydrofolate reductase inhibitorand to valuable intermediates in this process.
Owner:EVOLVA SA
Heterocyclic analogs of propargyl-linked inhibitors of dihydrofolate reductase
ActiveUS8853228B2Increase capacityStraightforward to synthesizeAntibacterial agentsOrganic active ingredientsBacteroidesProtozoa
Owner:UNIV OF CONNECTICUT
Pharmaceutical composition effective in preventing the adverse effects associated with the prolonged use of dihydrofolate reductase inhibitors
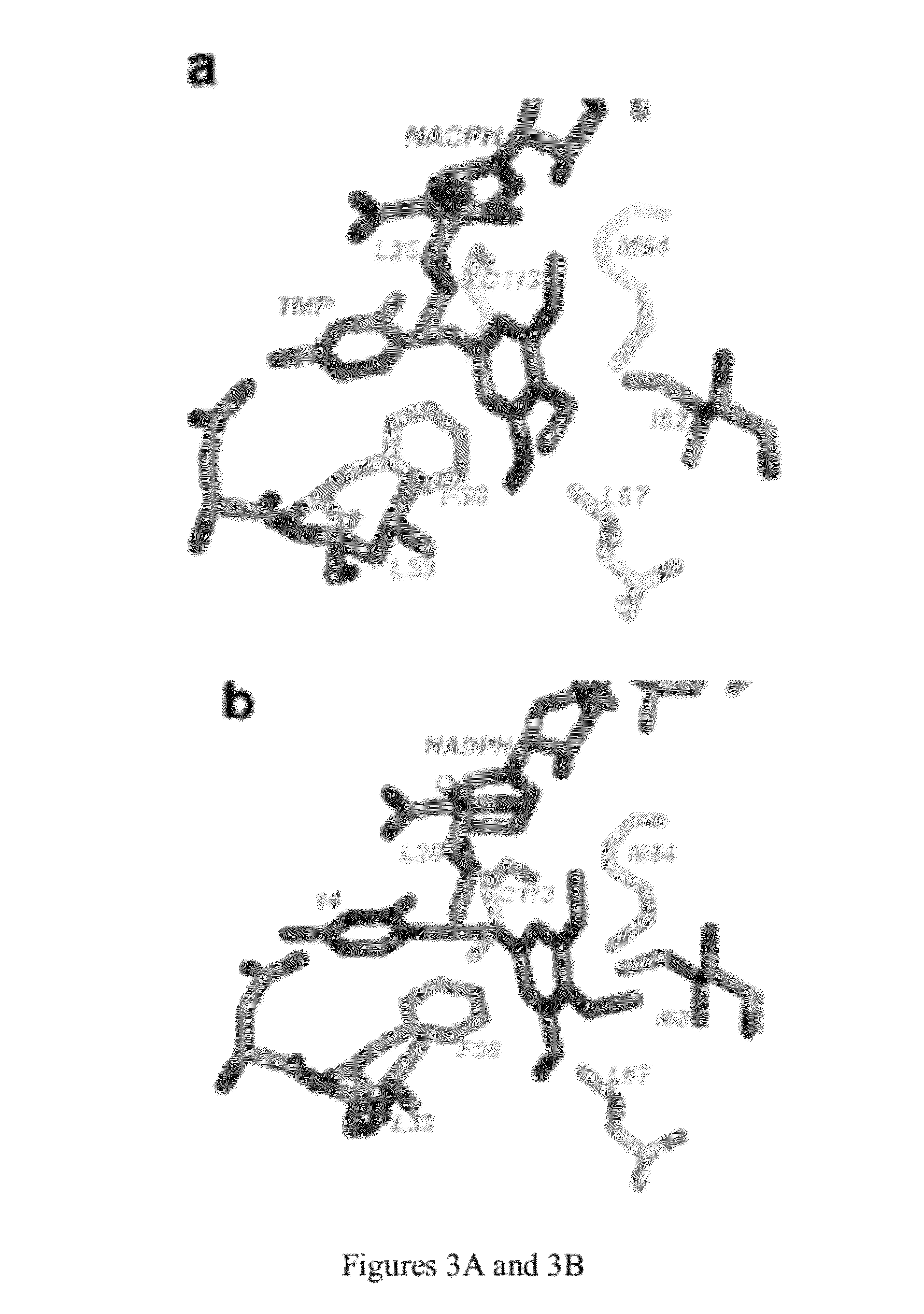
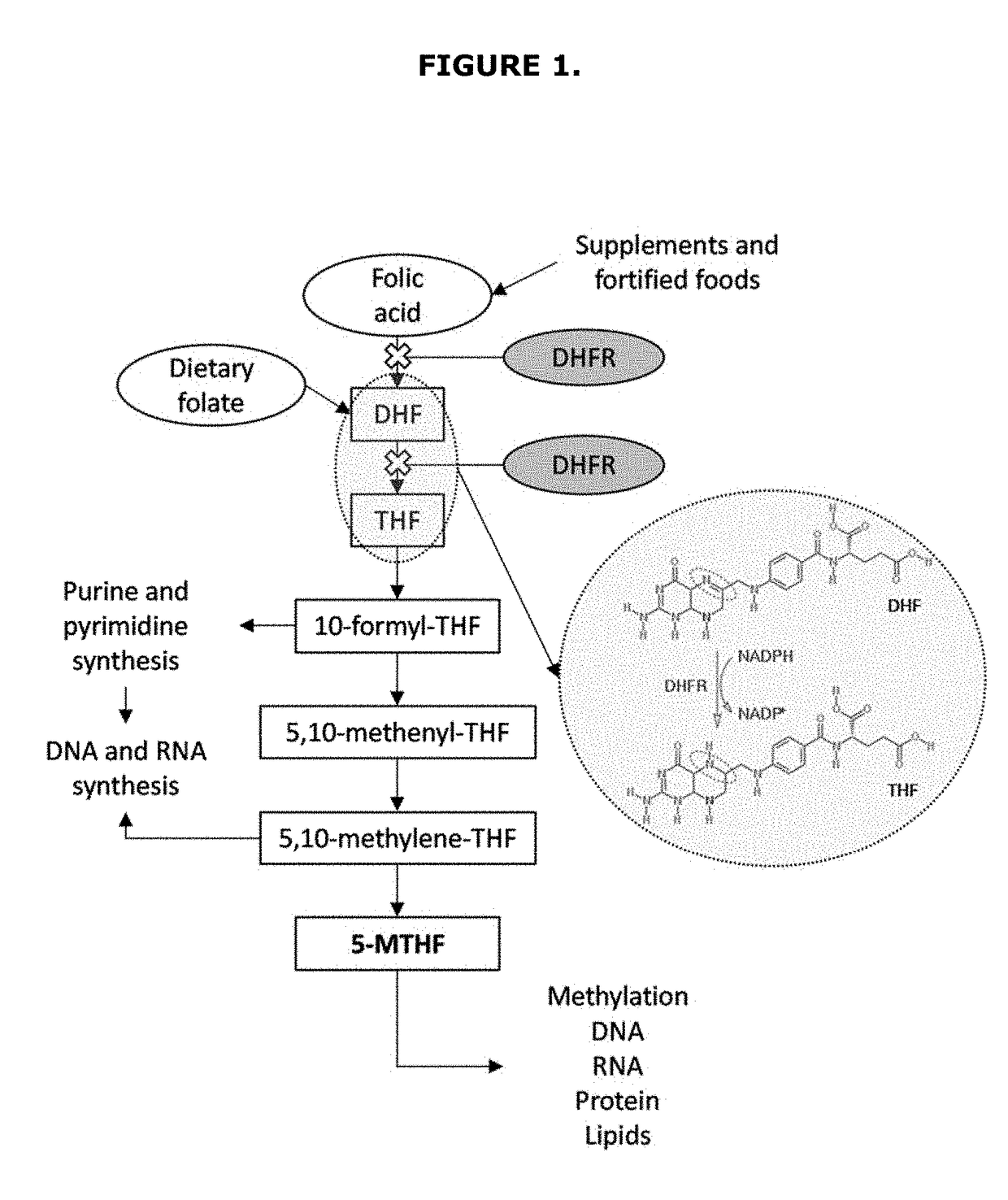
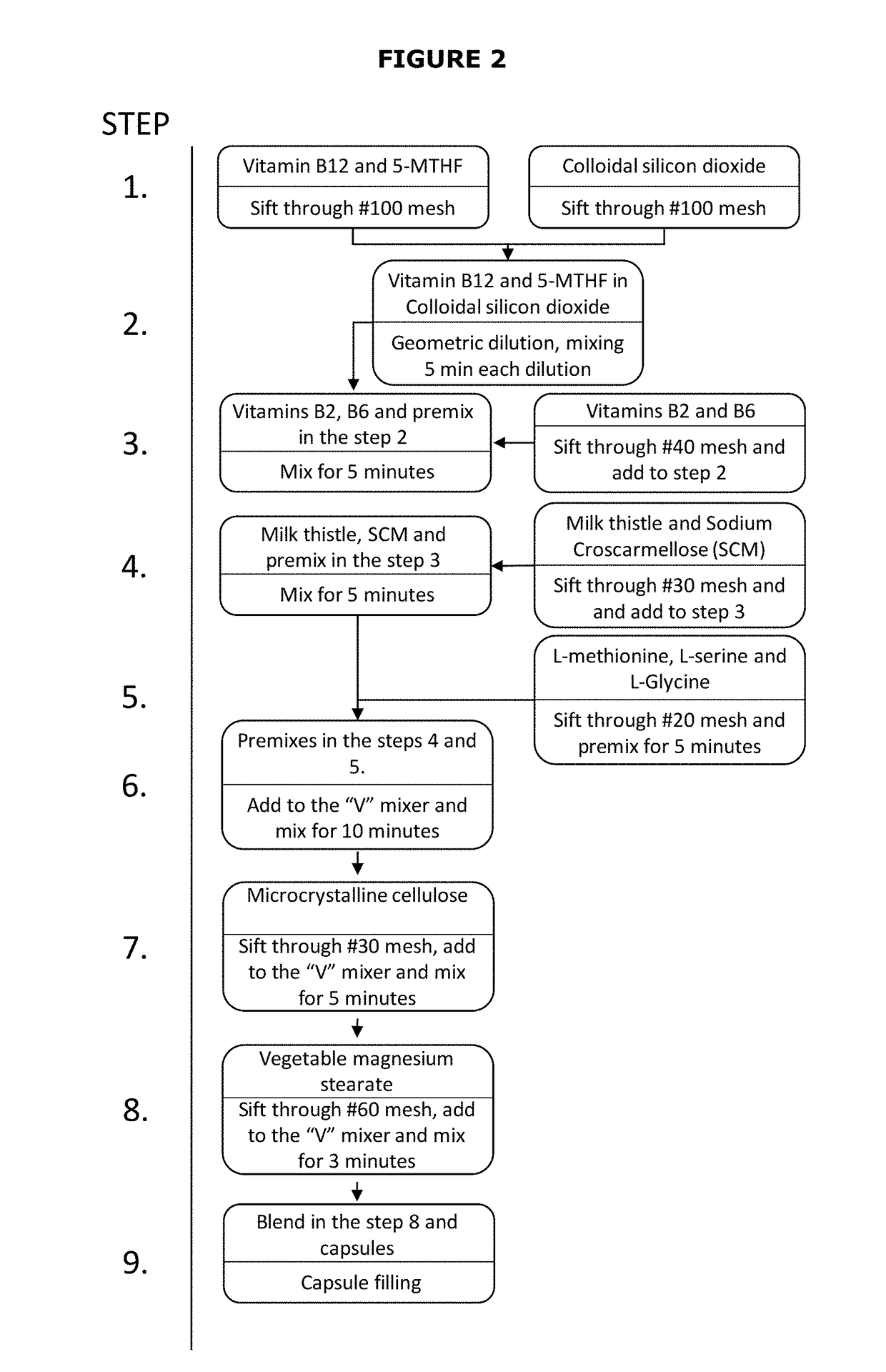
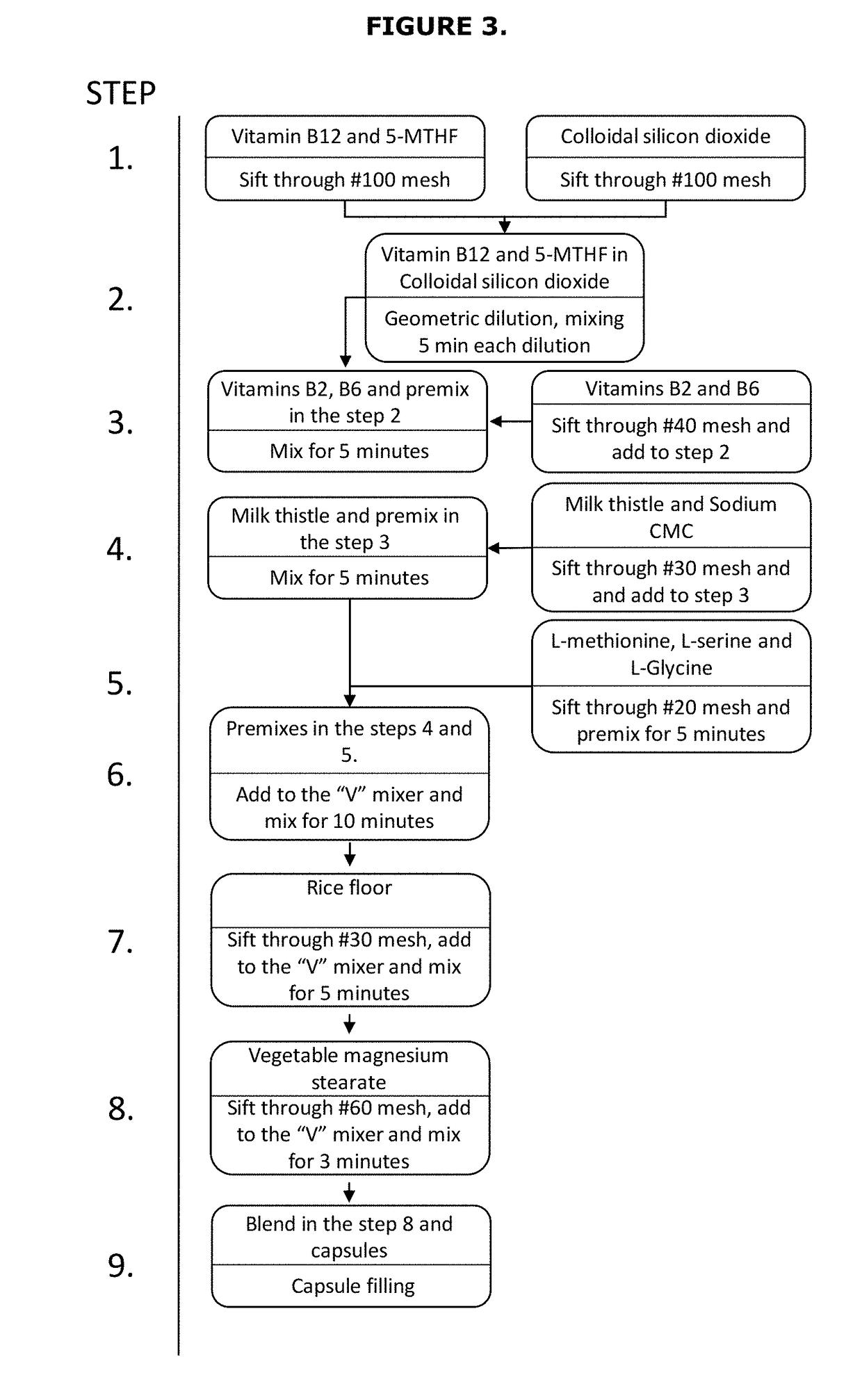
ActiveUS20170319586A1Reduce adverse effectsReduced form requirementsOrganic active ingredientsCapsule deliverySide effectEssential amino acid
The present invention relates to pharmaceutical compositions containing a combination of reduced forms of folate, liver protectors, vitamins, and essential or non-essential amino acids, useful in preventing the adverse effects associated with prolonged use of dihydrofolate reductase inhibitors.
Owner:COMPANION THERAPEUTICS LLC
Antiparasitic agent for fish and method of controlling proliferation of fish parasites
ActiveUS20120035181A1Imposes burdenResidue reductionBiocideOrganic chemistryAntiparasite agentOral medication
A method of controlling proliferation of fish parasites comprising the administration of 1 to 50 mg / kg fish body weight / day of an inhibitor of folate synthesis and / or an inhibitor of folate activation to fish continuously for 1 to 2 weeks. Using a combination preparation composed of an inhibitor of folate synthesis and an inhibitor of folate activation is preferable, and a sulfonamide is preferable for the inhibitor of folate synthesis. A dihydrofolate reductase inhibitor, a folate antagonist, etc., can be used as the inhibitor of folate activation. The antiparasitic agent is able to exterminate fish parasites via oral administration. It is particularly effective against parasites belonging to the ciliate group among fish parasites.
Owner:NIPPON SUISAN KAISHA LTD
Compositions and methods for treating infections
ActiveUS10774073B2Improve throughputLimit deliveryOrganic active ingredientsOrganic chemistryDihydrofolic acidPharmaceutical formulation
The invention relates to inhibitors of dihydrofolate reductase and pharmaceutical preparations thereof. The invention further relates to methods of treatment of parasitic infections, such as T. gondii, T. cruzi, P. falciparum, T. brucei, or L. major infections, using the novel inhibitors of the invention.
Owner:VYERA PHARMA LLC
Iron carrier-dihydrofolate reductase inhibitor conjugate and application thereof
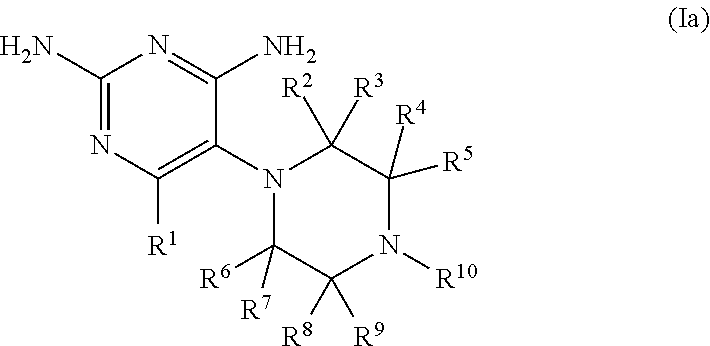
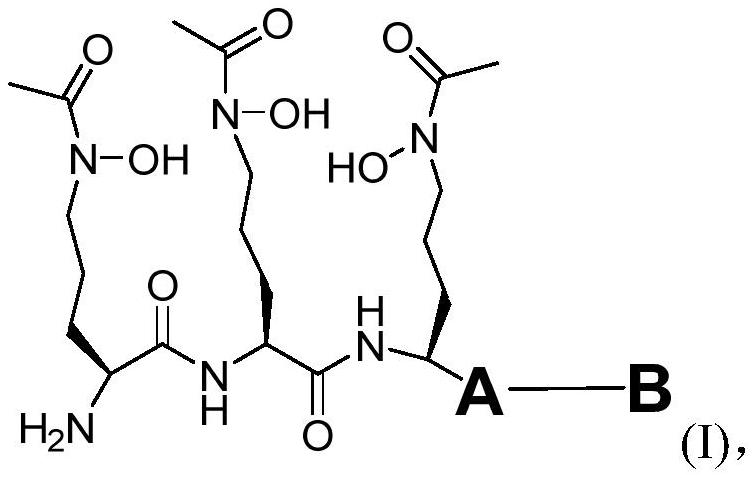
The invention provides a coupling compound which is a compound as shown in a formula (I) or a stereoisomer, a tautomer, a homologue, a solvate, a metabolite, a pharmaceutically acceptable salt or a prodrug of the compound as shown in the formula (I), A is a linking group, and B is a dihydrofolate reductase inhibitor.
Owner:JASAN BIO MEDICINE JIAXING CO LTD
Novel Processes For The Preparation Of A 2H-Chromene
The present invention relates to novel processes for the preparation of the compound of formula I (Iclaprim), related to dihydrofolate reductase inhibitorsand to valuable intermediates of these processes.
Owner:ARPIDA AG
Dihydrofolate reductase inhibitors
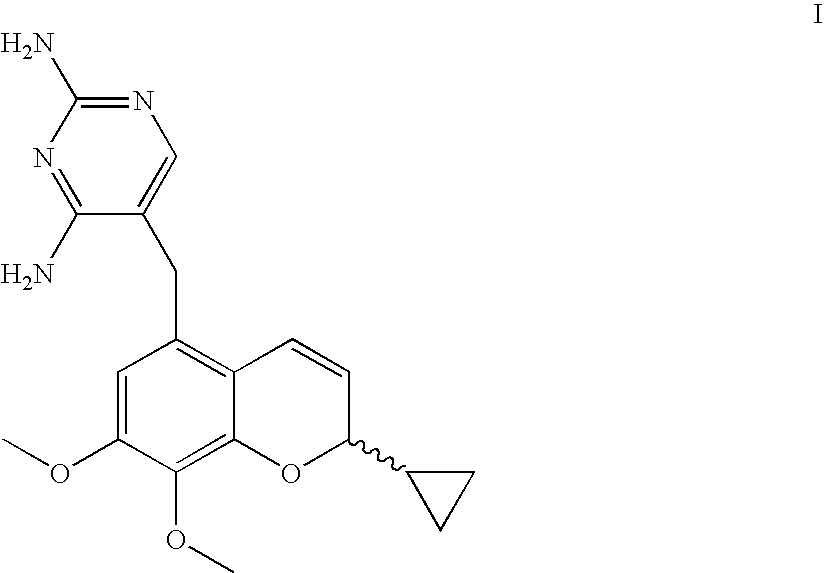
The present disclosure provides compounds of Formula I:or a pharmaceutically acceptable salt thereof, wherein R5, R6 and Z are as described herein. The disclosure also provides pharmaceutical compositions thereof; and methods for inhibiting DHFR activity; and methods for treating cell proliferative diseases, autoimmune disease, inflammatory disease or bacterial, fungal or parasitic infection by administering a compound of Formula I.
Owner:MERCK SHARP & DOHME LLC
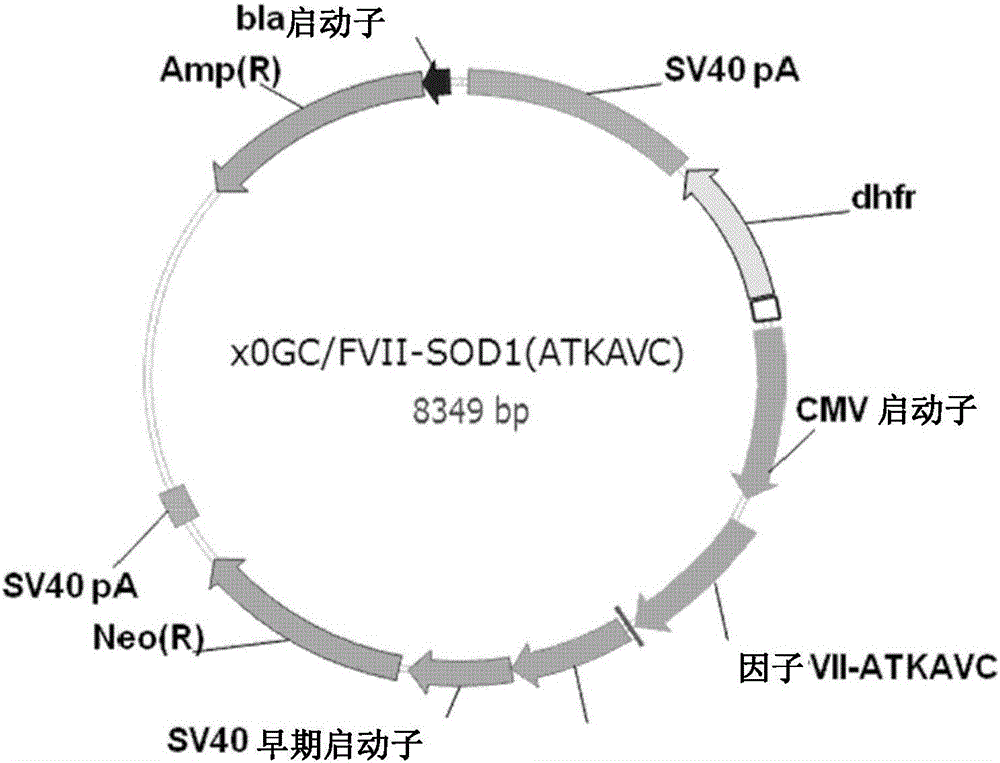
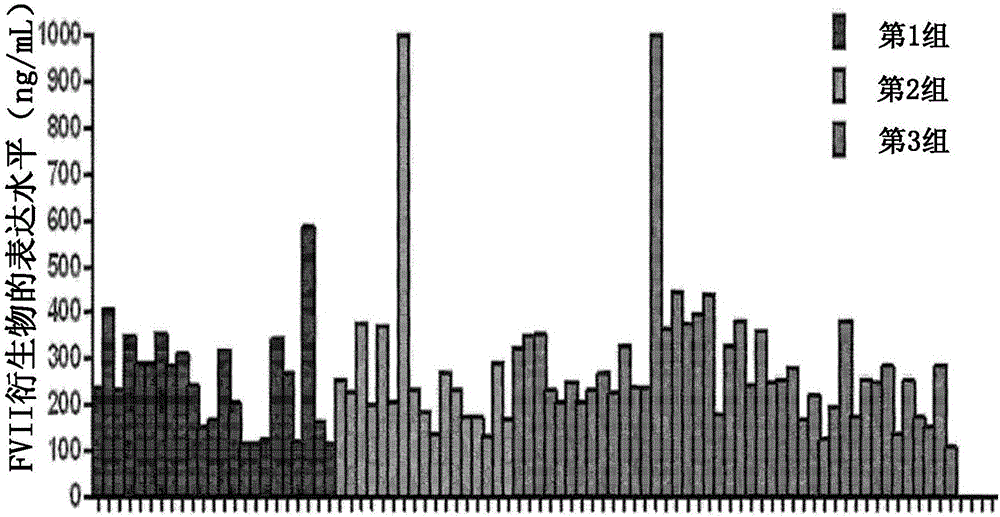
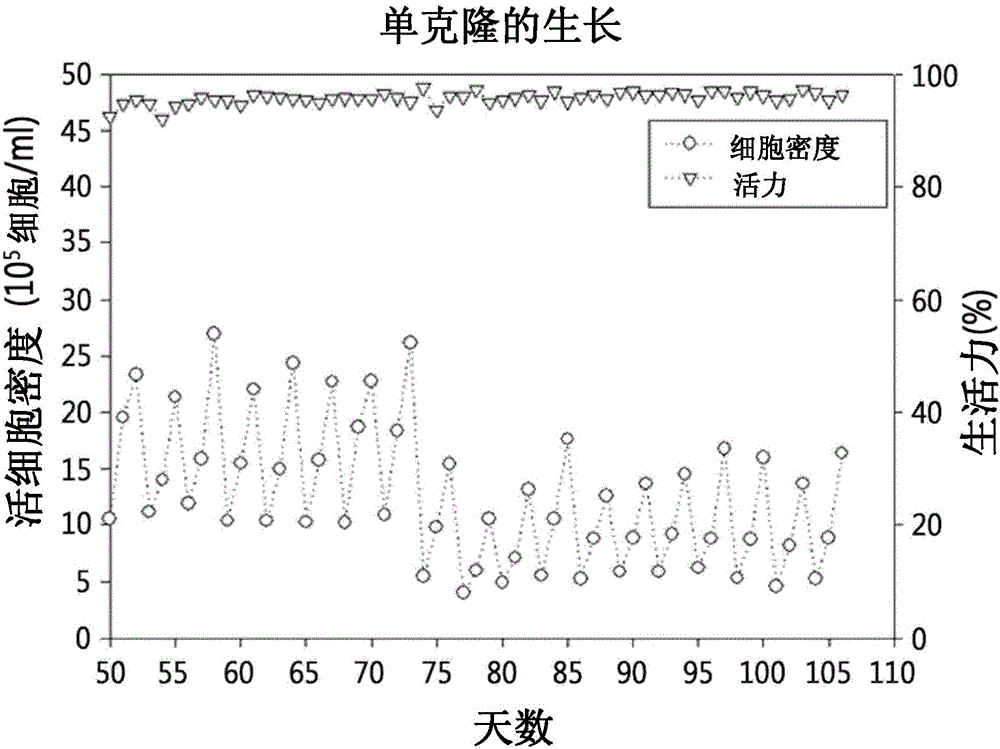
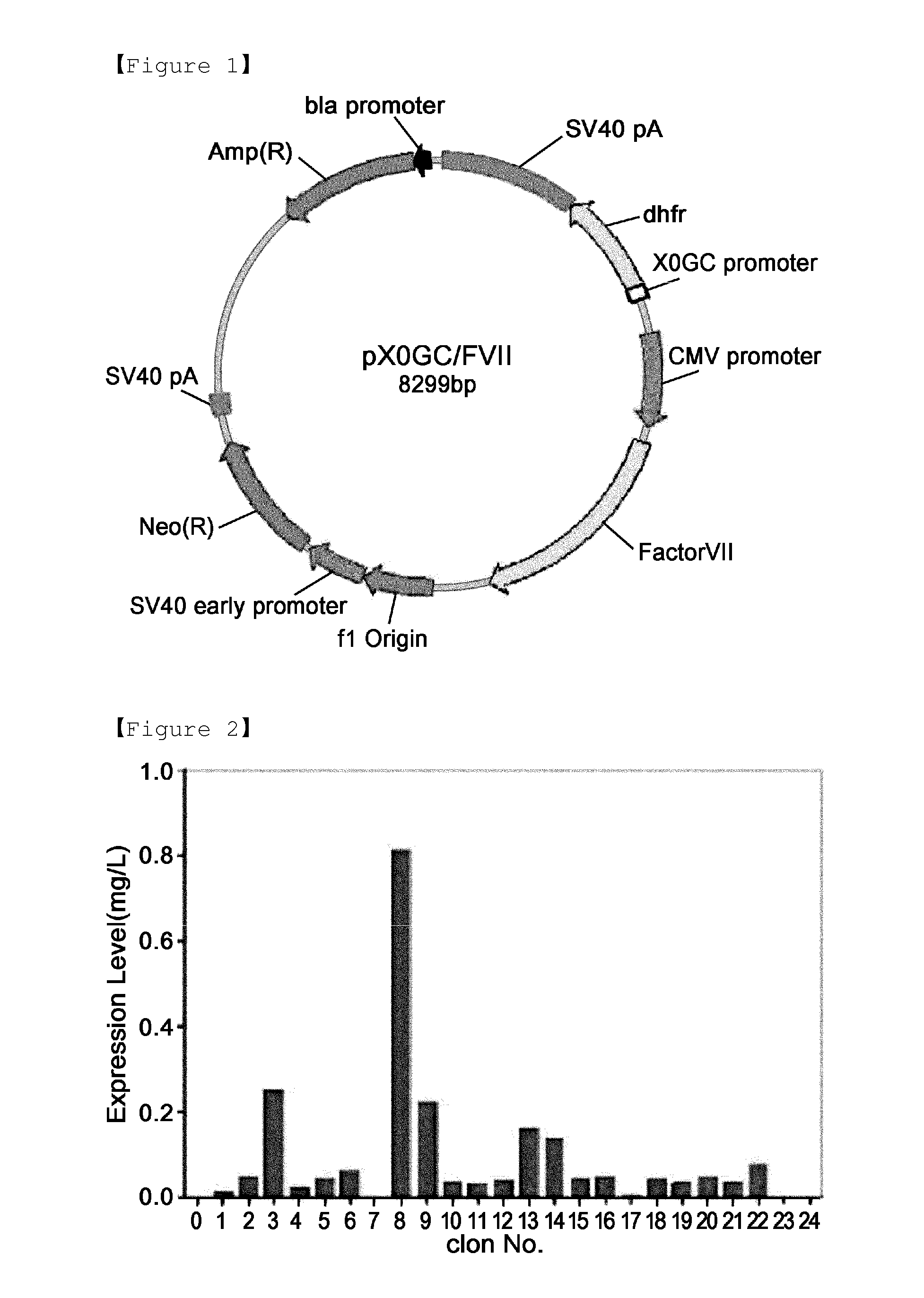
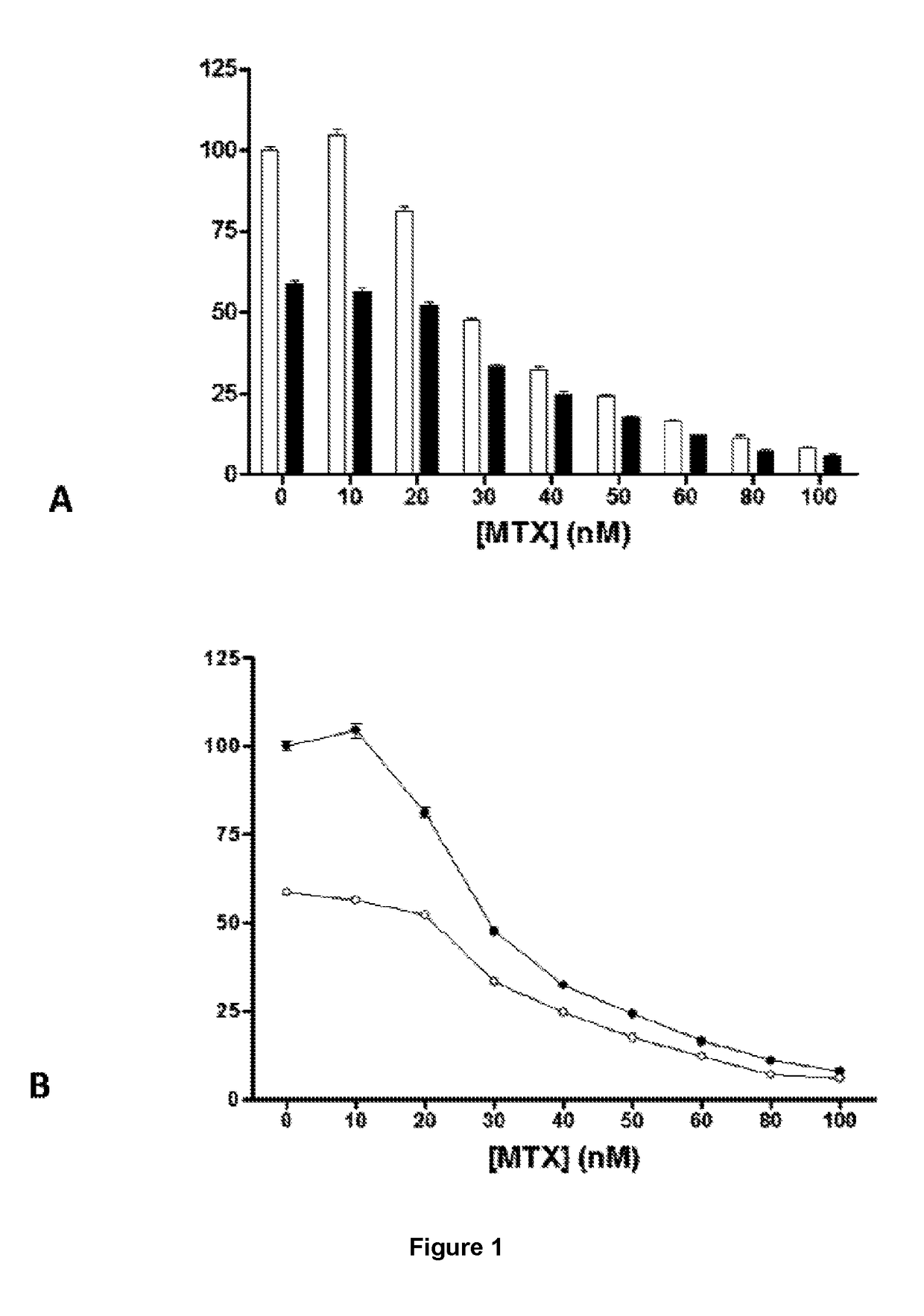
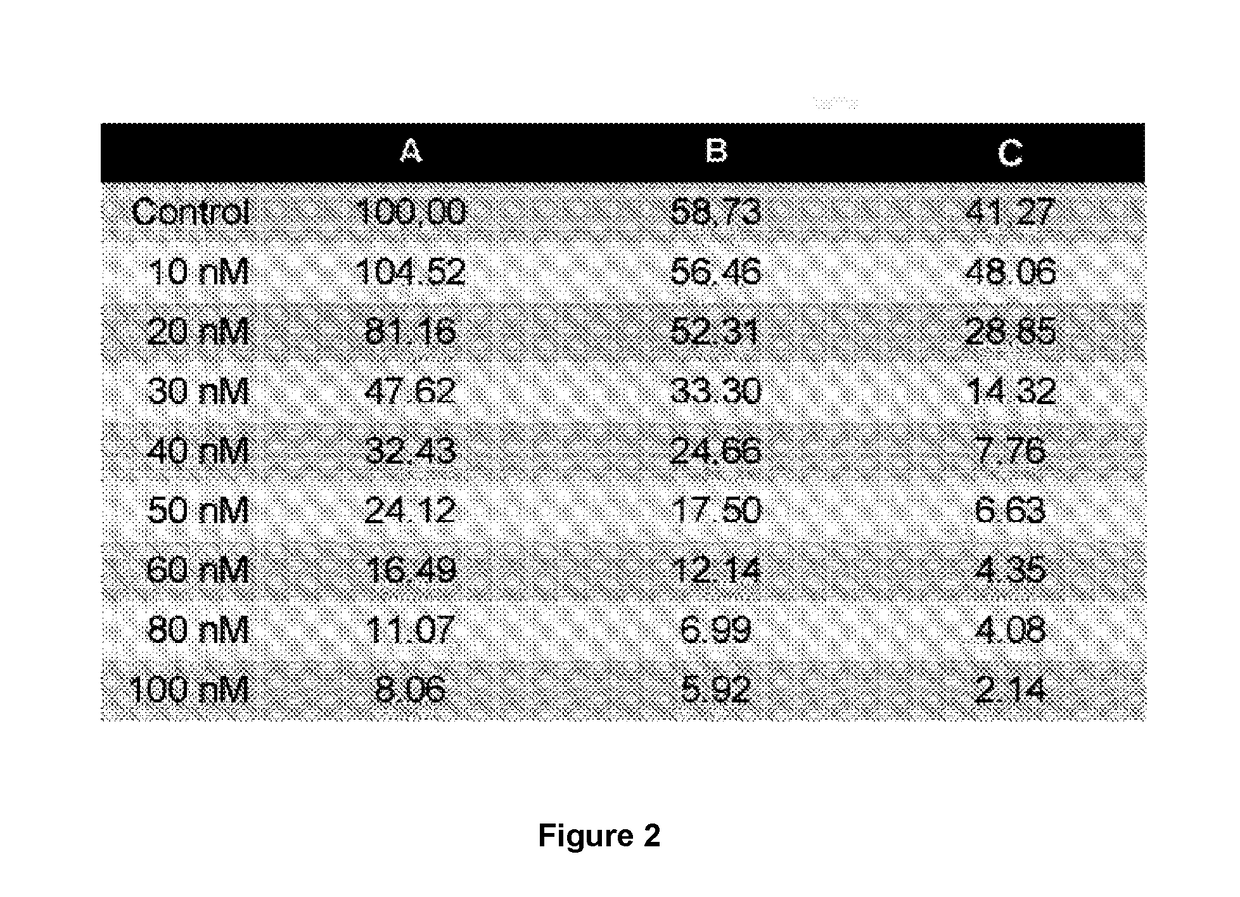
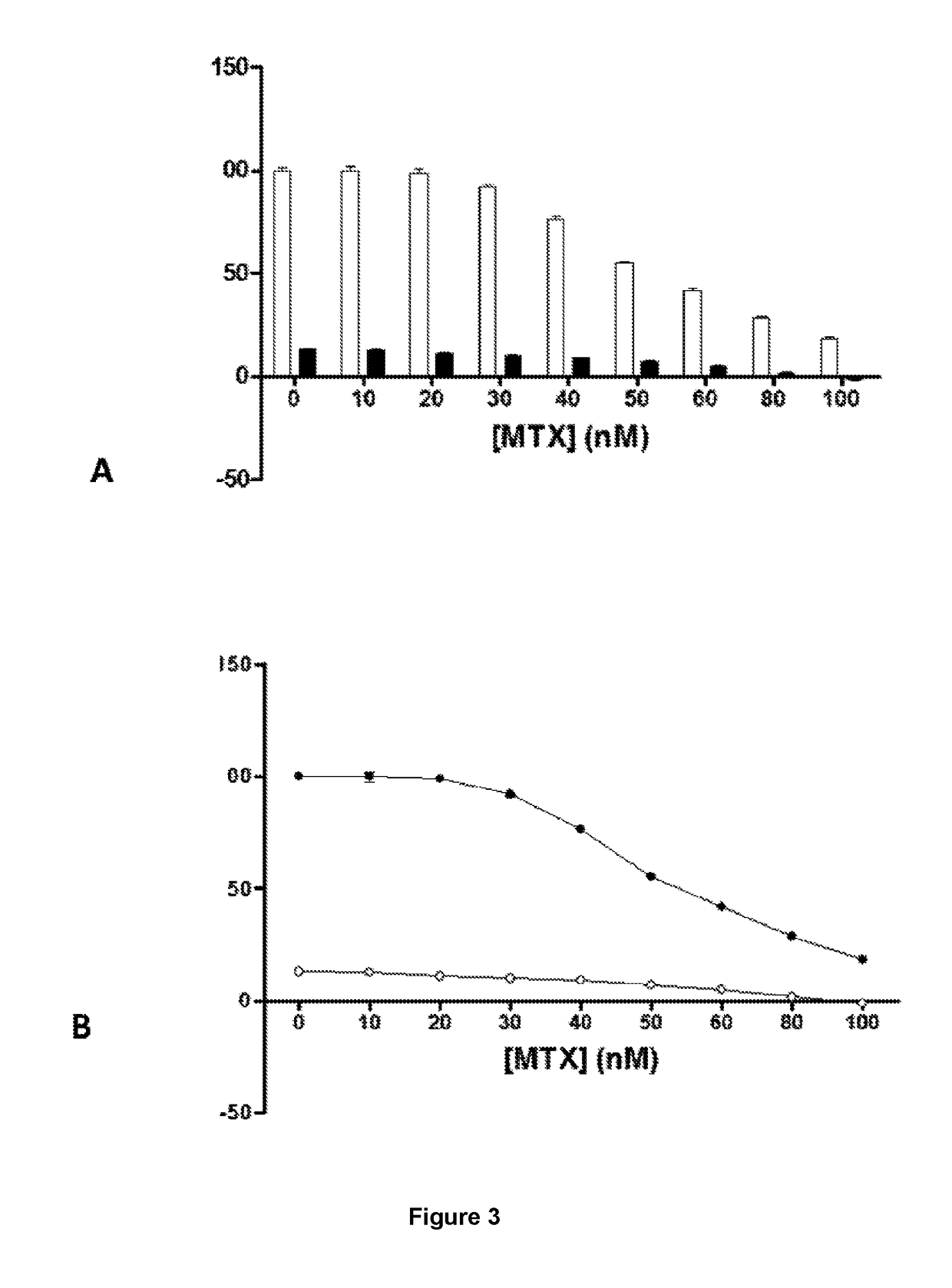
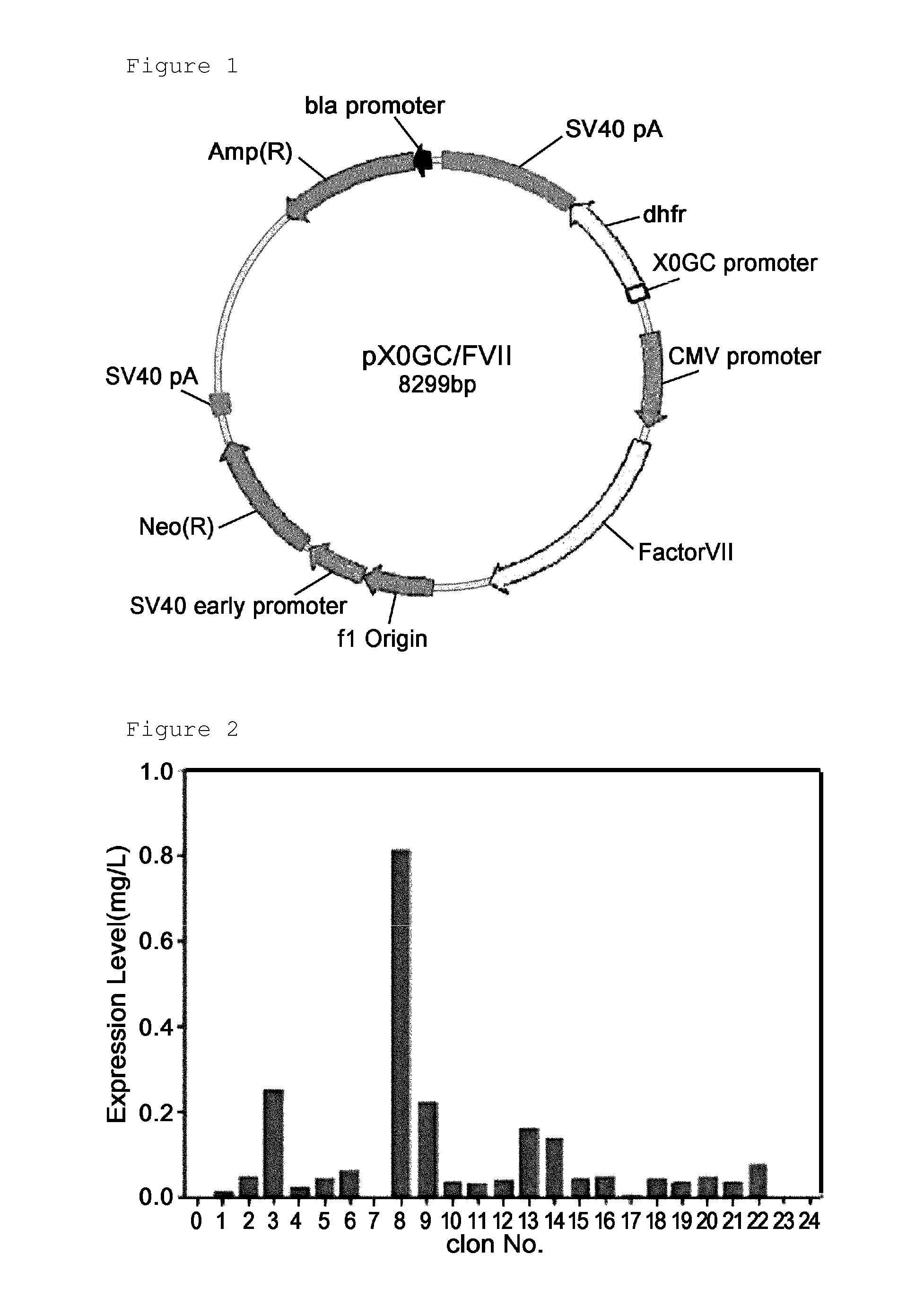
Method for mass producing human blood coagulation factor vii derivative
The present invention relates to a method for mass producing human blood coagulation factor VII, and more specifically, to a method for mass producing human blood coagulation factor VII, and a cell line for mass production of a human blood coagulation factor VII derivative, the method comprising the steps of: a) preparing an expression vector comprising i) a base sequence of dihydrofolate reductase (DHFR) promoter in which at least one CCGCCC is removed in a GC-rich region and a base sequence encoding DHFR operably linked thereto, and ii) a base sequence of an early gene of cytomegalovirus (CMV) and a base sequence encoding a human blood coagulation factor VII derivative operably linked thereto; b) transforming an animal cell line by using the expression vector of step; c) selecting a cell line expressing a human blood coagulation factor VII derivative with high efficiency by culturing the transformed animal cell line of step b) in the presence of a DHFR inhibitor; and d) culturing the selected animal cell line of step c) by adding at least one selected from the group consisting of sodium butyrate, vitamin K and a culture medium additive. The present invention can express a human blood coagulation factor VII derivative with high efficiency and in a large quantity by using a vector in which GC-rich repeating sequences are deleted in a DHFR promoter region, and thus can be usefully applied to the preparation of a therapeutic agent for hemophilia.
Owner:HANMI PHARMA
2,4-Diamino quinazoline and pyridopyrimidine ester derivatives as dihydrofolate reductase inhibitors
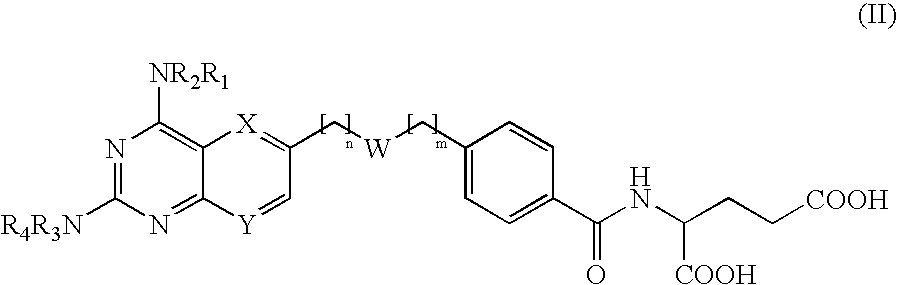
InactiveUS20060111376A1Increase productionIncreased formationBiocideOrganic active ingredientsElectron donorDihydrofolate reductase inhibitor
The invention provides novel compounds or the formula II: wherein R1, R2, R3 and R4 are independently hydrogen of a group that liberates the free amine in vivo, for example —CO-alkyl, preferably —CO—C1-C3 alkyl or pivalate; or —COhaloalkyl, preferably —CO—C1-C3 haloalkyl, most preferably —CO—C1-C3 chloroalkyl; wherein W is: and @ denotes the points of attachment and wherein the ester can be located in either direction; wherein n and m are independently 0-5; wherein one but not both or X and Y can be nitrogen, or X is C-A and / or Y is C—B; wherein A and B are independently selected from hydrogen, alkyl optionally substituted with a halogen, an electron donor group such as amino, alkylamino, dialkylamino or hydroxy, or an electron acceptor group such as nitro, cyano. trihaloalkyl or amido, alkoxy or halogen; and pharmacologically acceptable salts thereof. Provided that when R1 to R4 are hydrogen, both X and Y are C—H, n is 1 and —(CH2)n— is attached to the bridging oxygen of the ester group W, then m cannot be 0 or 1.
Owner:MELACURE THERAPEUTICS AB
Dhfr inhibitors, compositions, and methods related thereto
InactiveUS20200270233A1Improve throughputLimit deliveryOrganic chemistryAntiparasitic agentsMalarial parasiteDHFR Inhibitor
The invention relates to inhibitors of dihydrofolate reductase and pharmaceutical preparations thereof. The invention further relates to methods of treatment of parasitic infections, such as T gondii, T. cruzi, P. falciparum, T. brucei, or L. major infections, using the novel inhibitors of the invention.
Owner:VYERA PHARMA LLC
Method for mass production of factor vii/viia
A method for the mass production of human coagulation factor VII. The method includes a) providing an expression vector carrying i) a dihydrofolate reductase promoter devoid of one or more CCGCC repeat sequences from the GC-rich region thereof and a dihydrofolate reductase (DHFR) gene operably linked thereto and ii) a cytomegalovirus (CMV) promoter and a human coagulation factor VII gene operably linked thereto; b) obtaining a transformed a host cell line containing the expression vector; and c) culturing the transfected host cell in the presence of a dihydrofolate reductase inhibitor to select cells which express human coagulation factor VII with high efficiency; and d) adding sodium butyrate to the selected host cells.
Owner:HANMI SCI CO LTD
Boosting the effect of methotrexate through the combined use with lipophilic statins
The invention relates to the use of an inhibitor of the dihydrofolate reductase enzyme selected from the group that consists of methotrexate, trimetrexate and pemetrexed; or a pharmaceutically acceptable salt thereof, for the preparation of a drug for the treatment or prevention of recurrences of a disease selected from the group that consists of cancer, psoriasis, psoriatic arthritis, juvenile polyarticular arthritis, rheumatoid arthritis, Crohn's disease, polymyositis, dermatomyositis and sarcoidosis, wherein said treatment or prevention includes administering to a patient, simultaneously, separately or sequentially, a lipophilic statin and the inhibitor of the dihydrofolate reductase enzyme. The invention also relates to a pharmaceutical composition which includes the inhibitor of the dihydrofolate reductase enzyme and the lipophilic statin together with pharmaceutically acceptable carriers and / or vehicles.
Owner:DALANA3
Method for mass production of factor VII/VIIA
A method for the mass production of human coagulation Factor VII. The method includes a) providing an expression vector carrying i) a dihydrofolate reductase promoter devoid of one or more CCGCC repeat sequences from the GC-rich region thereof and a dihydrofolate reductase (DHFR) gene operably linked thereto and ii) a cytomegalovirus (CMV) promoter and a human coagulation Factor VII gene operably linked thereto; b) obtaining a transformed host cell line containing the expression vector; and c) culturing the transfected host cell in the presence of a dihydrofolate reductase inhibitor to select cells which express human coagulation Factor VII with high efficiency; and d) adding sodium butyrate to the selected host cells.
Owner:HANMI SCI CO LTD
Diaminodihydrotriazine derivative, its salt, preparation method, composition and application
ActiveCN103664972BStrong inhibitory activityBroaden research ideasAntibacterial agentsOrganic chemistryDiseaseDihydrofolic acid
The invention discloses derivatives and salts of damino dihydrotriazine, and a preparation method, a composition and application thereof. According to the invention, the preparation method of the damino dihydrotriazine derivative and the damino dihydrotriazine salt can be realized by adopting a method I or a method II, wherein the method I includes the step of obtaining a general formula I compound prepared through the reaction between a general formula IV compound and a general formula V compound, while the method II includes the step of mixing a general formula VIII compound with a general formula II compound under an acidic condition, and obtaining the compound shown in the general formula I through a cyclization reaction of the mixture. The invention also provides application of derivatives and salts of the damino dihydrotriazine in preparation of human dihydrofolate reductase inhibitors, preventing and curing drugs for tumor or bacterial infection diseases. The invention further provides a drug composition, which comprises an effective amount of the derivatives and / or salts of the damino dihydrotriazine, as well as pharmaceutically acceptable carriers. According to the invention, spiro heterocyclic ring derivatives of the damino dihydrotriazine have an excellent inhibitory activity on human dihydrofolate reductase, tumor cells and bacteria.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Compositions and methods for treating infections
ActiveUS20190077794A1Improve throughputLimit deliveryOrganic active ingredientsOrganic chemistryMedicineDihydrofolate reductase inhibitor
The invention relates to inhibitors of dihydrofolate reductase and pharmaceutical preparations thereof. The invention further relates to methods of treatment of parasitic infections, such as T. gondii, T. cruzi, P. falciparum, T. brucei, or L. major infections, using the novel inhibitors of the invention.
Owner:VYERA PHARMA LLC
Boosting the effect of methotrexate through the combined use with lipophilic statins
InactiveUS20160175310A1Less side effectsBiocideAntipyreticDihydrofolate reductase inhibitorSarcoidosis
The invention relates to the use of an inhibitor of the dihydrofolate reductase enzyme selected from the group that consists of methotrexate, trimetrexate and pemetrexed; or a pharmaceutically acceptable salt thereof, for the preparation of a drug for the treatment or prevention of recurrences of a disease selected from the group that consists of cancer, psoriasis, psoriatic arthritis, juvenile polyarticular arthritis, rheumatoid arthritis, Crohn's disease, polymyositis, dermatomyositis and sarcoidosis, wherein said treatment or prevention includes administering to a patient, simultaneously, separately or sequentially, a lipophilic statin and the inhibitor of the dihydrofolate reductase enzyme. The invention also relates to a pharmaceutical composition which includes the inhibitor of the dihydrofolate reductase enzyme and the lipophilic statin together with pharmaceutically acceptable carriers and / or vehicles.
Owner:DALANA3
Antiparasitic agent for fish and method of controlling proliferation of fish parasites
An antiparasitic agent for fish includes an inhibitor of folate synthesis and / or an inhibitor of folate activation as the active substance(s). A combination preparation composed of an inhibitor of folate synthesis and an inhibitor of folate activation is disclosed. Sulfonamide is disclosed as suitable for the inhibitor of folate synthesis. A dihydrofolate reductase inhibitor, a folate antagonist are disclosed as suitable inhibitors of folate activation. The antiparasitic agent is able to exterminate fish parasites via oral administration. It is effective against fish parasites belonging to the ciliate group.
Owner:NIPPON SUISAN KAISHA LTD
A combined drug with antitumor drug efficacy
ActiveCN107496399BSmall toxicitySignificant effectOrganic active ingredientsAntineoplastic agentsChlorogenic acidSide effect
The invention provides a combined medicine having efficacy of antineoplastic drugs. The combined medicine consists of chlorogenic acid and a dihydrofolate reductase inhibitor of unit preparations in same or different specifications, and a pharmaceutically acceptable carrier, wherein the chlorogenic acid and the dihydrofolate reductase inhibitor are applied in a simultaneous or separated mode. According to the combined medicine, a synergistic effect can be developed when the chlorogenic acid and the DHFR (dihydrofolate reductase) inhibitor are used in a combined mode, and hematopoietic function injury and weight loss caused by the DHFR inhibitor can be relieved; toxic and side effects of the DHFR inhibitor can be effectively reduced; and the medicine, which combines the chlorogenic acid and the DHFR inhibitor is excellent in curative effect, low in toxicity and good in prospect of clinical application.
Owner:SICHUAN JIUZHANG BIO TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com