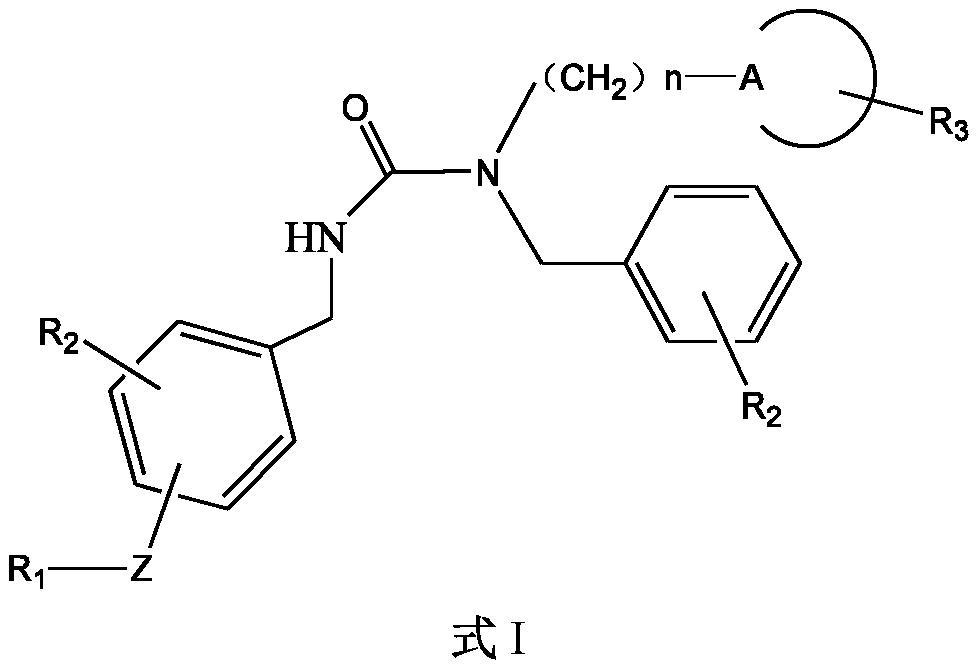
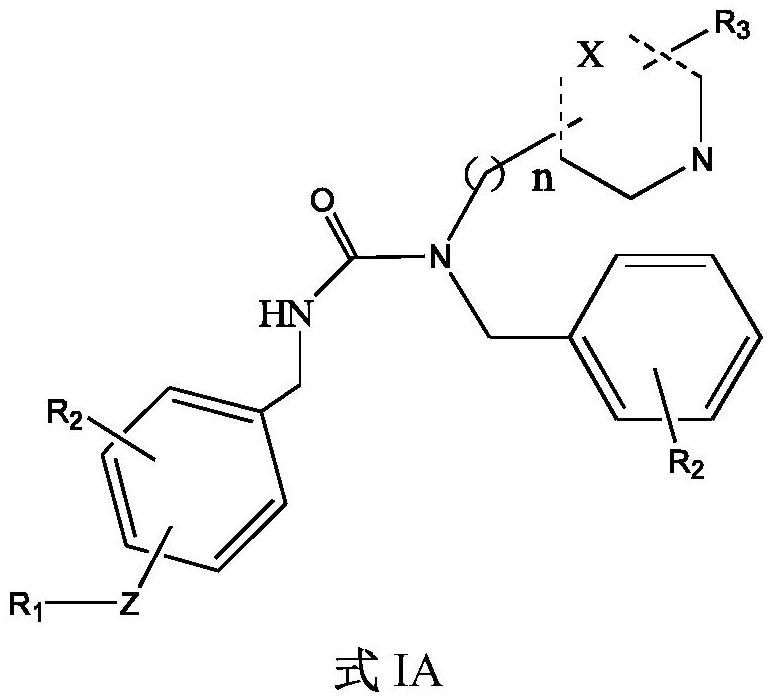
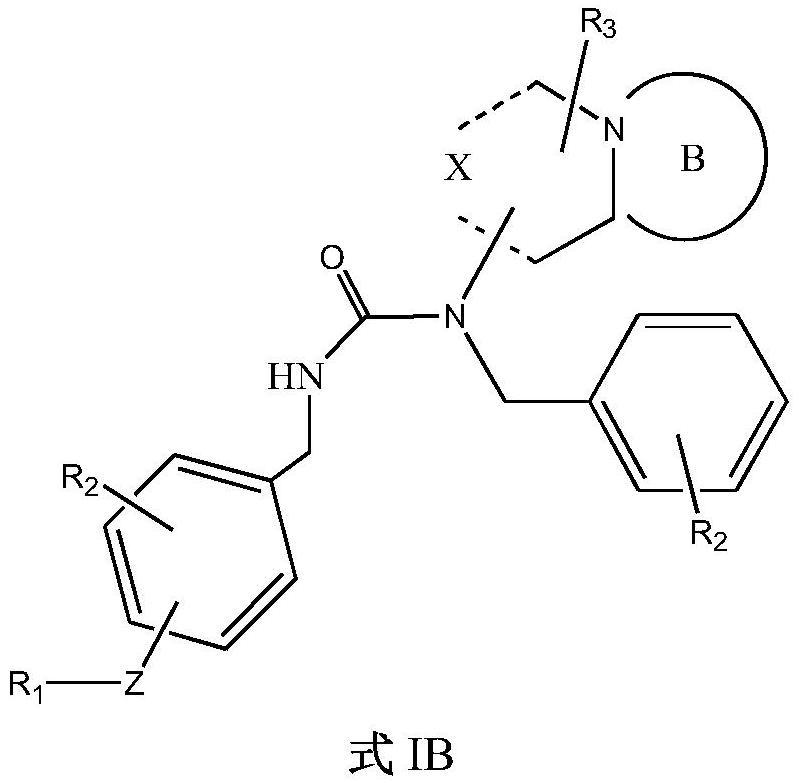
5-HT2A receptor antagonist, and application thereof in treating central nervous system diseases
A solvate, selected technology, applied in the field of medicine, can solve the problems of type 2 diabetes, abnormal motor function and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0173] Example 1: Synthesis of 1-(4-fluoro-benzyl)-3-(4-isobutoxy-benzyl)-1-(9-methyl-3-oxa-9-aza- Bicyclo[3.3.1]non-7-yl)-urea (Compound#1), ER10048
[0174]
[0175] (4-fluoro-benzyl)-(9-methyl-3-oxa-9-za-bicyclo[3,3,1]non-7-yl)amine (1-1): at room temperature, 4-Fluorobenzene Methylamine (151mg, 1.21mmol), then sodium triacetoxyborohydride (341mg, 1.61mmol), and 0.1ml of acetic acid were added slowly in portions. The mixture was stirred overnight at room temperature. Ice water (10 ml) was added, and the mixture was extracted with 10% (v / v) isopropanol / chloroform (30 ml×2). Organic Phase Na 2 SO 4 Drying, filtration and concentration under vacuum gave Intermediate (1-1) (181 mg, 85% yield) as a colorless oil. LCMS: [M+1] + 265.2.
[0176]
[0177] 4-Isobutoxybenzonitrile (1-2): In acetone (150 mL), potassium carbonate (58.0 g, 420 mmol) was added to 4-hydroxybenzonitrile (25.0 g, 210 mmol) and 1-bromo -2-Methylpropane (34.5g, 252mmol) in a mixture. Stir at 60...
Embodiment 2
[0184] Example 2: Synthesis of 1-(3-(azetidin-1-yl)-propyl)-1-(4-fluoro-benzyl)-3-(4-isobutoxy-benzyl) - Urea (Compound #12), ER10053
[0185]
[0186] (3-(azetidin-1)-yl-propyl)-(4-fluoro-benzyl)-amine (12-1): The synthesis method is similar to intermediate (1-1). Using 0.88 mmol of 3-(azetidin-1-yl)-propylamine and 4-fluorobenzaldehyde, compound (12-1) (155 mg, 80% yield) was obtained as a brown oil. LCMS: [M+1] + 223.3.
[0187]
[0188] 1-(3-(azetidin-1-yl)-propyl)-1-(4-fluoro-benzyl)-3-(4-isobutoxy-benzyl)-urea (Compound# 12) The synthesis method is similar to compound#1. With 0.70mmol (3-(azetidin-1)-yl-propyl)-(4-fluoro-benzyl)-amine (12-1), and 1-isobutoxy-4-(isocyanate Acidomethyl)benzene (1-4) was reacted to give compound #12 (100 mg. 33% yield) as a white solid. 1 H NMR (300MHz, CDCl3): δ7.31(d, J=6.4Hz, 4H), 7.01(t, J=8.7Hz, 2H), 6.93-6.84(m, 2H), 4.48(s, 2H), 4.41(s, 2H), 3.73(d, J=6.5Hz, 2H), 3.245(s, 2Hz), 3.02-2.91(m, 4H), 2.36(d, J=5.9Hz, 2H), 2.0...
Embodiment 3
[0189] Example 3: Synthesis of 1-(4-fluoro-benzyl)-3-(4-isobutoxy-benzyl)-1-(1-methyl-pyrrolidin-3-ylmethyl)- Urea (compound#10), ER10054
[0190]
[0191] (4-Fluoro-benzyl)-(1-methyl-pyrrolidin-3-ylmethyl)-amine (10-1): The synthesis method is similar to intermediate (1-1). (1-Methyl-pyrrolidin-3-yl)-methanamine (100 mg, 0.87 mmol) was reacted with 4-fluorobenzaldehyde to give product (10-1) (155 mg, 80% yield) as yellow oil. LCMS: [M+1] + 223.4.
[0192]
[0193] 1-(4-Fluoro-benzyl)-3-(4-isobutoxy-benzyl)-1-(1-methyl-pyrrolidin-3-ylmethyl)-urea (compound#10 ) synthesis method is similar to compound#1. With (4-fluoro-benzyl)-(1-methyl-pyrrolidin-3-ylmethyl)-amine (10-1) 0.69mmol and 1-isobutoxy-4-(isocyanato Methyl)benzene (1-4) was reacted to give compound #10 (140 mg, 47% yield) as a yellow solid. 1 H NMR (300MHz, CDCl 3 ): δ7.29-7.17(m, 4H), 7.02(dd, J=9.8, 7.5Hz, 2H), 6.86(dd, J=9.1, 2.4Hz, 2H), 6.37(s, 1H), 4.70- 4.58(m, 2H), 4.44-4.32(m, 2H), 3.80(s, 2H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com