Preparation method of end group functionalized hyperbranched polyolefin
A polyolefin and functional polymer technology, applied in the field of polyolefin synthesis, can solve the problems of increased synthesis process, practical application limitations, limited group types, etc., and achieve the effect of improving production capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] This example relates to a method for synthesizing end group-functionalized hyperbranched polyolefins with controllable branching degree and controllable end group types. The synthesis route is as follows: figure 1 As shown, it specifically includes the following steps:
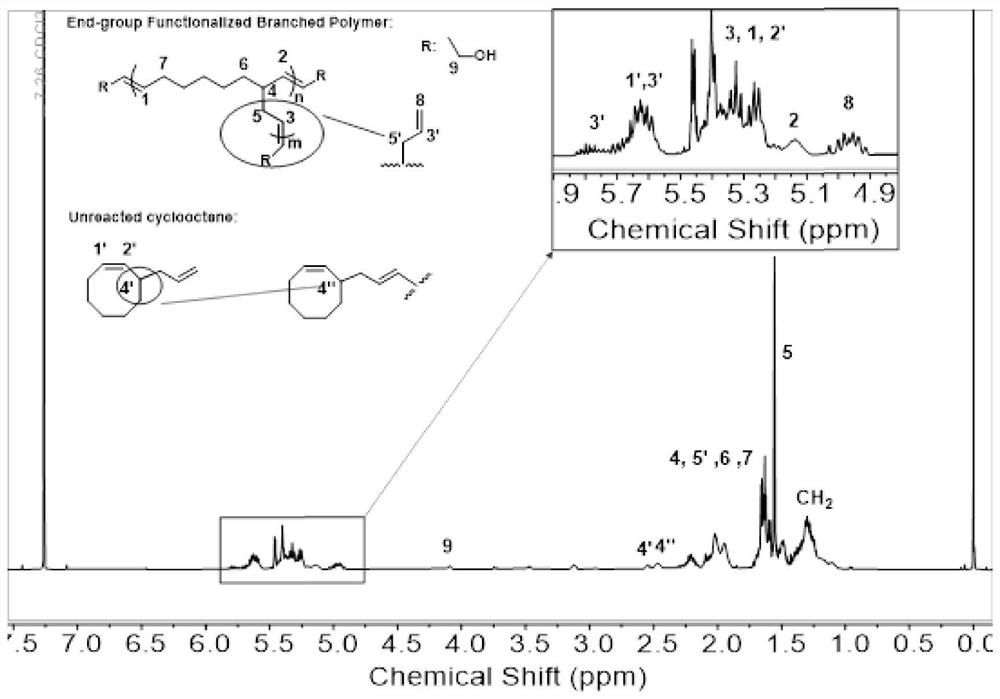
[0038] Take 3-allyl-cyclooctene (1mmol in 1ml tetrahydrofuran), put it in a 2ml sample bottle, add Hoveyda-Grubbs second-generation catalyst (3.13mg, dissolved in 20μL tetrahydrofuran) and chain transfer agent cis-2 -Butene-1,4-diol (4.41mg, dissolved in 20μL THF), reacted under argon for 24h (reaction temperature was 50°C), and then added vinyl ether / THF solution (v:v=50% )0.5ml to terminate the reaction, the product structure is as figure 2 Shown: n is 8, m is 1, the conversion rate of the monomer is 54.8%, and the branching degree of the product is 29.9%.
Embodiment 2
[0040]This example relates to a method for synthesizing end group-functionalized hyperbranched polyolefins with controllable branching degree and controllable end group types. The synthesis route is as follows: figure 1 As shown, it specifically includes the following steps:
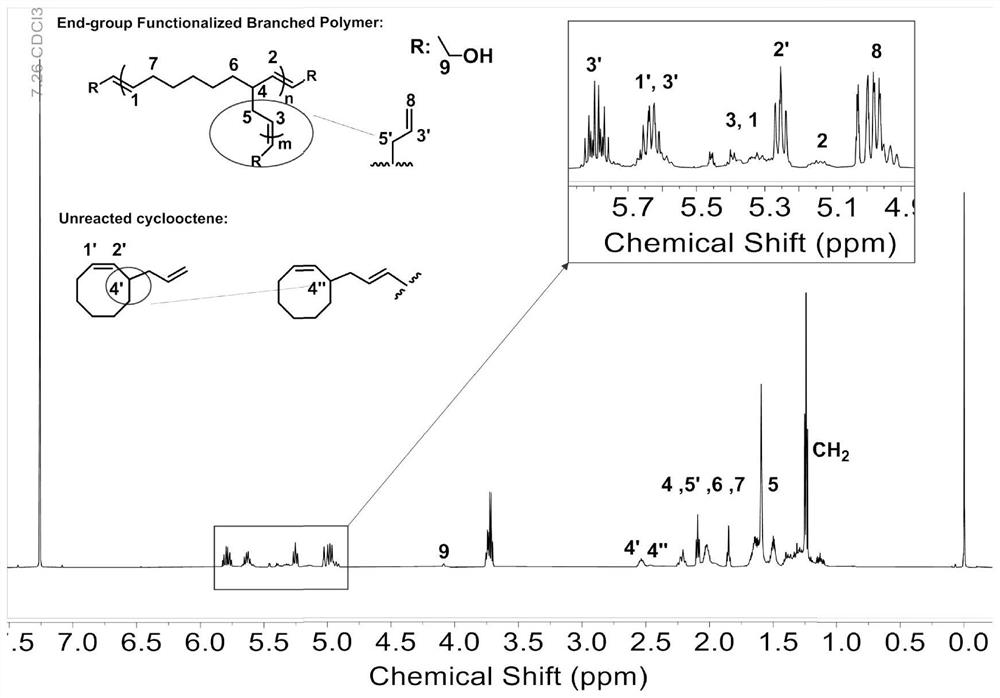
[0041] Take 3-allyl-cyclooctene (1mmol in 1ml tetrahydrofuran), put it in a 2ml sample bottle, add Grubbs third-generation catalyst (4.42mg, dissolved in 20μL tetrahydrofuran) and chain transfer agent cis-2-butane Alkene-1,4-diol (4.41 mg, dissolved in 20 μL THF) was reacted under argon for 24 h (reaction temperature was 25° C.), and then vinyl ether / THF solution (v:v=50%) 0.5 ml to terminate the reaction, the product structure is as follows image 3 Shown: n is 10, m is 1, the conversion rate of the monomer is 42.6%, and the branching degree of the product is 14.9%.
Embodiment 3
[0043] This example relates to a method for synthesizing end group-functionalized hyperbranched polyolefins with controllable branching degree and controllable end group types. The synthesis route is as follows: figure 1 As shown, it specifically includes the following steps:
[0044] Take 3-allyl-cyclooctene (1mmol in 1ml THF), put it in a 2ml sample bottle, add Grubbs second-generation catalyst (4.43mg, dissolved in 20μL THF) and chain transfer agent maleic acid (17.41 mg, dissolved in 100 μL tetrahydrofuran), reacted under argon for 24h (reaction temperature was 50°C), then added 0.5ml of vinyl ether / tetrahydrofuran solution (v:v=50%) to terminate the reaction, and the product structure was as follows Figure 4 Shown: n is 6, m is 1, the conversion rate of the monomer is 91.8%, and the branching degree of the product is 15.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com