Synthesis Method of Hyperbranched Polyolefin with controllable branched chain length and controllable branching degree
A synthesis method and technology of polyolefins, applied in the field of polyolefins, can solve the problems that cannot be used to synthesize hyperbranched polyolefins, and achieve the effect of good fluidity and low viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
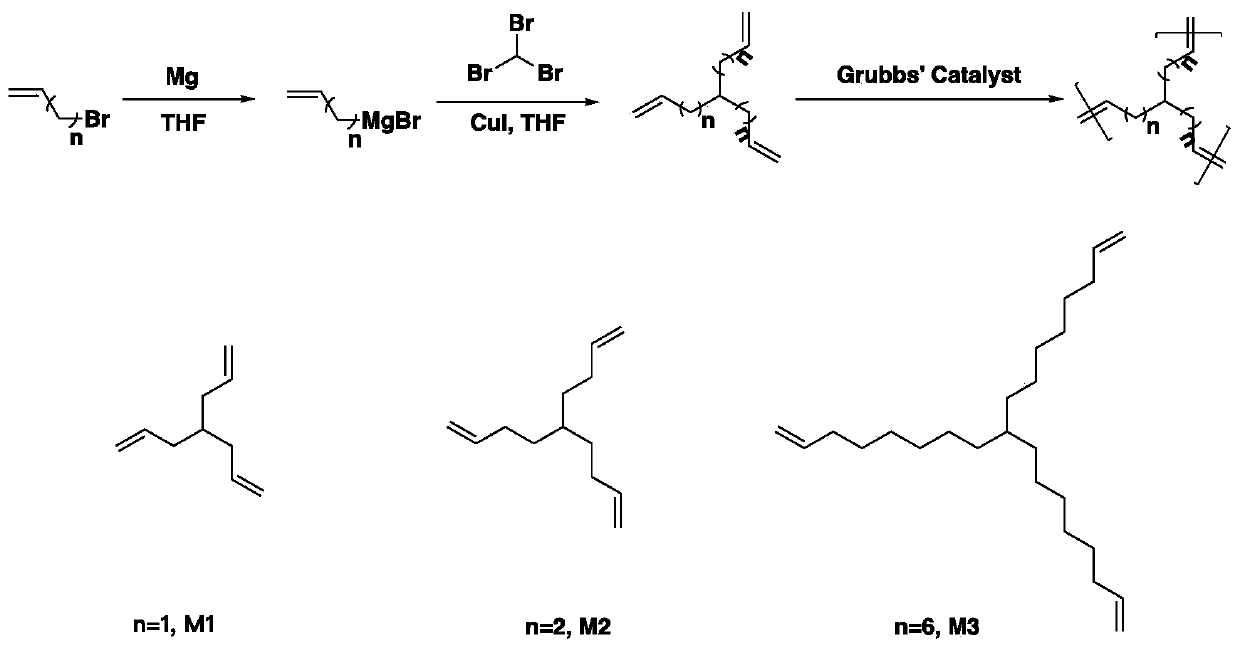
[0039] This embodiment relates to a method for synthesizing hyperbranched polyolefins with controllable branching chain length and controllable degree of branching. The synthetic route is as follows: figure 1 As shown, it specifically includes the following steps:
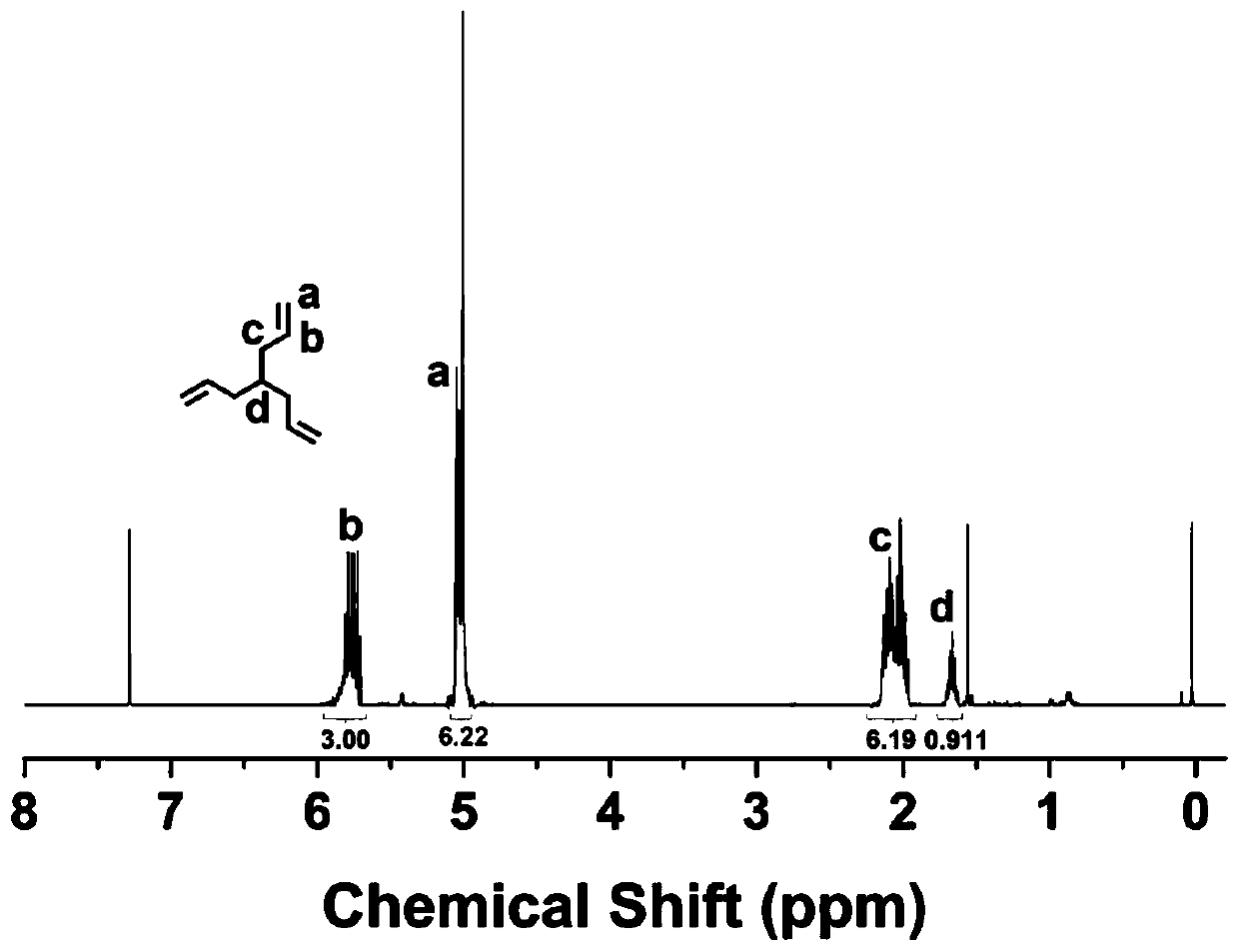
[0040] Under the protection of argon, add cuprous iodide (0.1g, 0.53mmol), bromoform (5g, 0.020mol), and tetrahydrofuran (50mL) into a 200mL reaction flask equipped with a magnetic stirrer, and slowly drip into it under an ice bath Allyl magnesium bromide solution (75 mL, 0.075 mol). After reacting for 3 hours, saturated ammonium chloride solution was added to terminate the reaction, then saturated brine was added, and the mixture was extracted three times with ether. The resulting solution was dried with anhydrous magnesium sulfate for 30 minutes and then concentrated by rotary evaporation to obtain a crude product. The crude product was purified by a silica gel column (mobile phase was n-hexane) to obtain a pure pr...
Embodiment 2
[0046] This embodiment relates to a method for synthesizing hyperbranched polyolefins with controllable branching chain length and controllable degree of branching. The synthetic route is as follows: figure 1 As shown, it specifically includes the following steps:
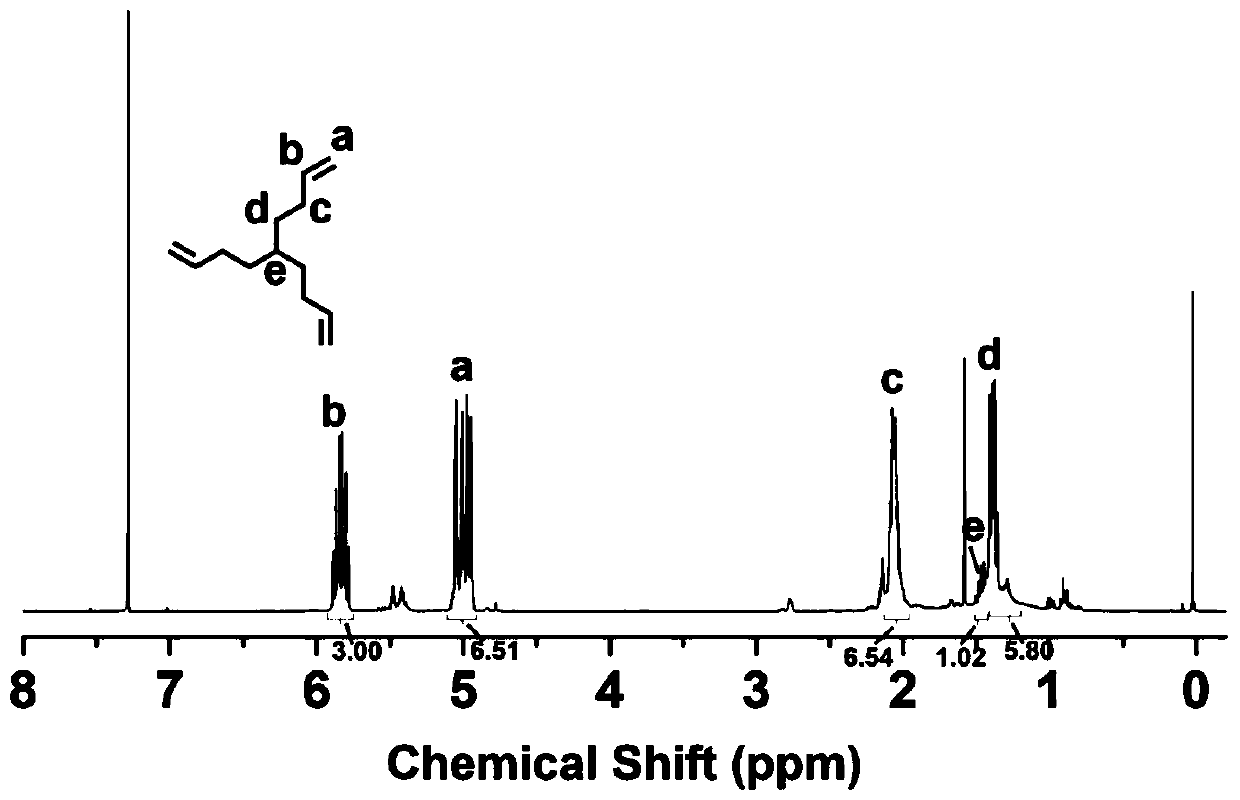
[0047] Under the protection of argon, add cuprous iodide (0.1g, 0.53mmol), bromoform (5g, 0.020mol), and tetrahydrofuran (50mL) into a 200mL reaction flask equipped with a magnetic stirrer, and slowly drip into it under an ice bath 1-Butene-4-magnesium bromide solution (200 mL, 0.075 mol). After reacting for 3 hours, saturated ammonium chloride solution was added to terminate the reaction, then saturated brine was added, and the mixture was extracted three times with ether. The resulting solution was dried with anhydrous magnesium sulfate for 30 minutes and then concentrated by rotary evaporation to obtain a crude product. The crude product was purified by silica gel column (mobile phase was n-hexane) to obtain pure ...
Embodiment 3
[0052] This embodiment relates to a method for synthesizing hyperbranched polyolefins with controllable branching chain length and controllable degree of branching. The synthetic route is as follows: figure 1 As shown, it specifically includes the following steps:
[0053] Under the protection of argon, add cuprous iodide (0.1g, 0.53mmol), bromoform (5g, 0.020mol), and tetrahydrofuran (50mL) into a 200mL reaction flask equipped with a magnetic stirrer, and slowly drip into it under an ice bath 1-Butene-4-magnesium bromide solution (200 mL, 0.075 mol). After reacting for 3 hours, saturated ammonium chloride solution was added to terminate the reaction, then saturated brine was added, and the mixture was extracted three times with ether. The resulting solution was dried with anhydrous magnesium sulfate for 30 minutes and then concentrated by rotary evaporation to obtain a crude product. The crude product was purified by silica gel column (mobile phase was n-hexane) to obtain pure ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com