Preparation method and packaging method of octreotide acetate injection
A technology of octreotide acetate and encapsulation method, which is applied in the field of pharmacy, can solve problems such as poor stability of octreotide acetate injection, and achieve the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
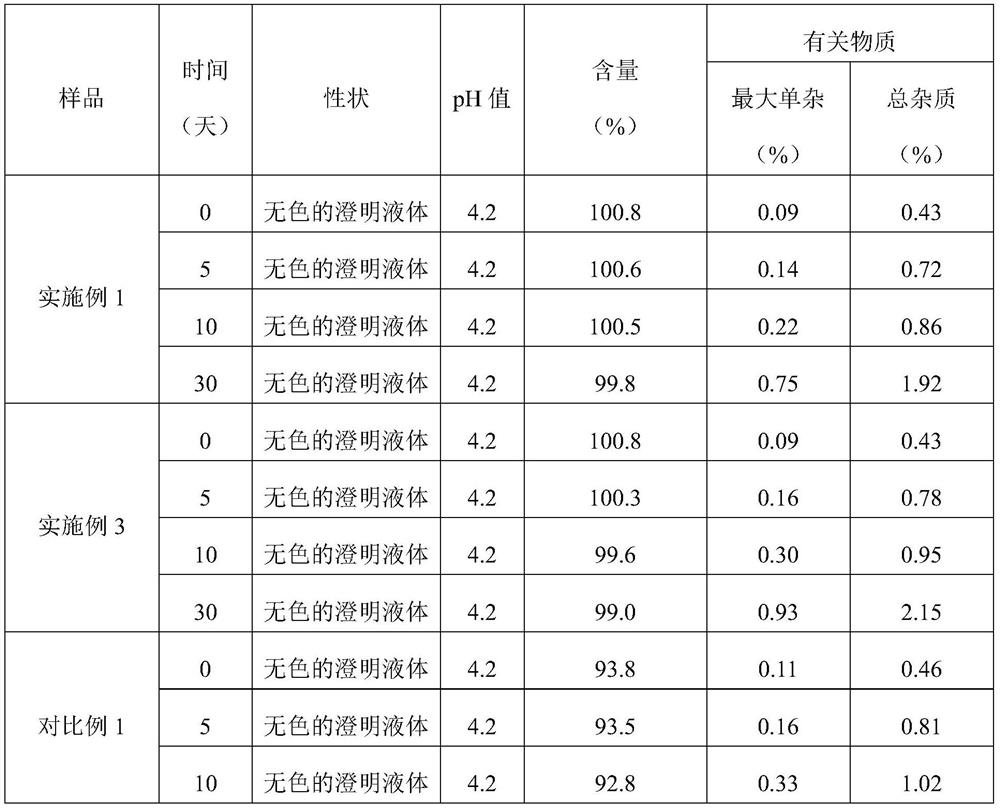
Embodiment 1
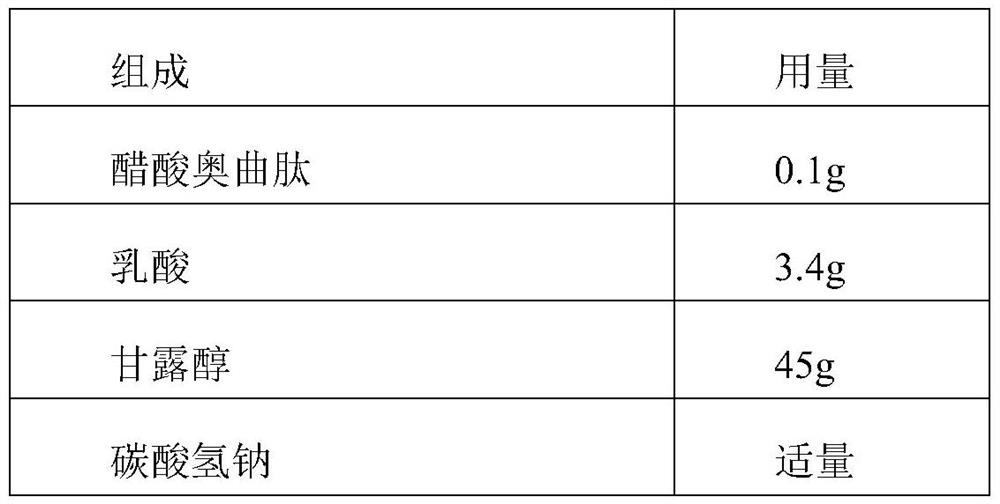
[0021] Table 1: Prescription
[0022] composition Dosage Octreotide Acetate 0.1g lactic acid 3.5g Mannitol 45g sodium bicarbonate Appropriate amount Add water for injection to 1L
[0023] Preparation Process:
[0024] Measure 850mL of water for injection (the water temperature is controlled at 5-25°C), add 3.5g of lactic acid, stir and dissolve evenly, add 45g of mannitol, stir and dissolve evenly, adjust the pH value of the above solution to 4.2 with 6% sodium bicarbonate solution, add acetic acid Octreotide 0.1g, stir until dissolved evenly, add water for injection to 1L. The medicinal solution is sterilized and filtered through a secondary 0.2μm filter element. The liquid medicine is filled in a 2mL medium borosilicate vial, filled with carbon dioxide, quickly corked and capped.
Embodiment 2
[0026] Table 2: Prescription
[0027] composition Dosage Octreotide Acetate 0.1g lactic acid 3.6g Mannitol 40g sodium bicarbonate Appropriate amount Add water for injection to 1L
[0028] Measure 850mL of water for injection (the water temperature is controlled at 5-25°C), add 3.6g of lactic acid, stir and dissolve evenly, add 40g of mannitol, stir and dissolve evenly, adjust the pH value of the above solution to 4.1 with 5% sodium bicarbonate solution, add acetic acid Octreotide 0.1g, stir until dissolved evenly, add water for injection to 1L. The medicinal solution is sterilized and filtered through a secondary 0.2μm filter element. The liquid medicine is filled in a 2mL medium borosilicate vial, filled with carbon dioxide, quickly corked and capped.
Embodiment 3
[0030] The preparation of octreotide acetate injection does not use the carbon dioxide protective gas process.
[0031] Table 3: Prescription
[0032] composition Dosage Octreotide Acetate 0.1g lactic acid 3.5g Mannitol 45g sodium bicarbonate Appropriate amount Add water for injection to 1L
[0033] Preparation Process:
[0034] Measure 850mL of water for injection (the water temperature is controlled at 5-25°C), add 3.5g of lactic acid, stir and dissolve evenly, add 45g of mannitol, stir and dissolve evenly, adjust the pH value of the above solution to 4.2 with 6% sodium bicarbonate solution, add acetic acid Octreotide 0.1g, stir until dissolved evenly, add water for injection to 1L. The medicinal solution is sterilized and filtered through a secondary 0.2μm filter element. The liquid medicine is filled in 2mL medium borosilicate vials, corked and capped.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com