Signal peptide for improving antibody yield
A signal peptide and antibody technology, applied in the field of molecular biology, can solve problems such as differences in antibody secretion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Construction of antibody fusion protein 3D6-SpyTag heavy chain expression vector
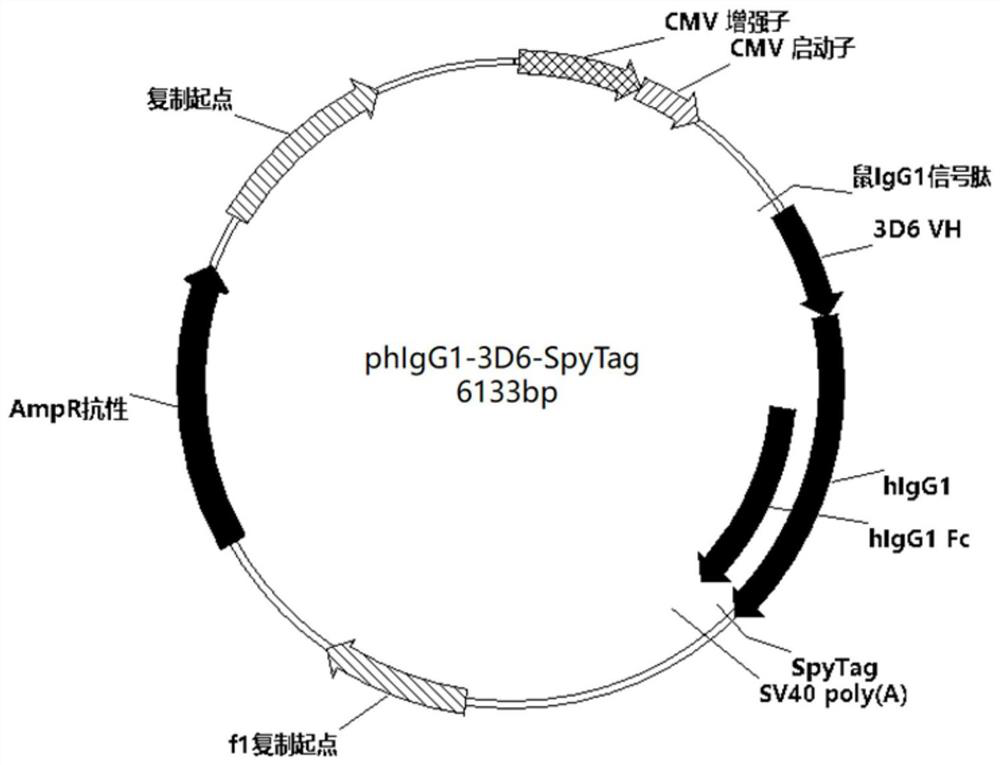
[0045]SpyTag (polypeptide segment) amino acid sequence (SEQ ID NO: 1) mammalian codon optimized sequence (SEQ ID NO: 2) and 3D6 (anti-human DEC205 monoclonal antibody) heavy chain expression vector phIgG1-3D6 (signal peptide is mouse IgG1 signal peptide, amino acid sequence SEQ ID NO: 3) and light chain expression vector phIgK-3D6 (signal peptide is mouse IgG1 signal peptide, amino acid sequence SEQ ID NO: 3) were provided by Suzhou Industrial Park Weida Biotechnology Co., Ltd. Using phIgG1-3D6 as a template, design primers (Table 1), amplify the hIgG1-SpyTag gene fragment by PCR, then recover the corresponding fragment from the gel, and clone it into the multiple cloning site between Sal I and HindIII on the phIgG1-3D6 vector. Construct the heavy chain expression vector phIgG1-3D6-SpyTag that can express fusion protein 3D6-SpyTag (plasmid map such as figure 1 ), which were cor...
Embodiment 2
[0050] Example 2 Construction of antibody fusion protein 3D6-SpyTag (N) heavy chain expression vector
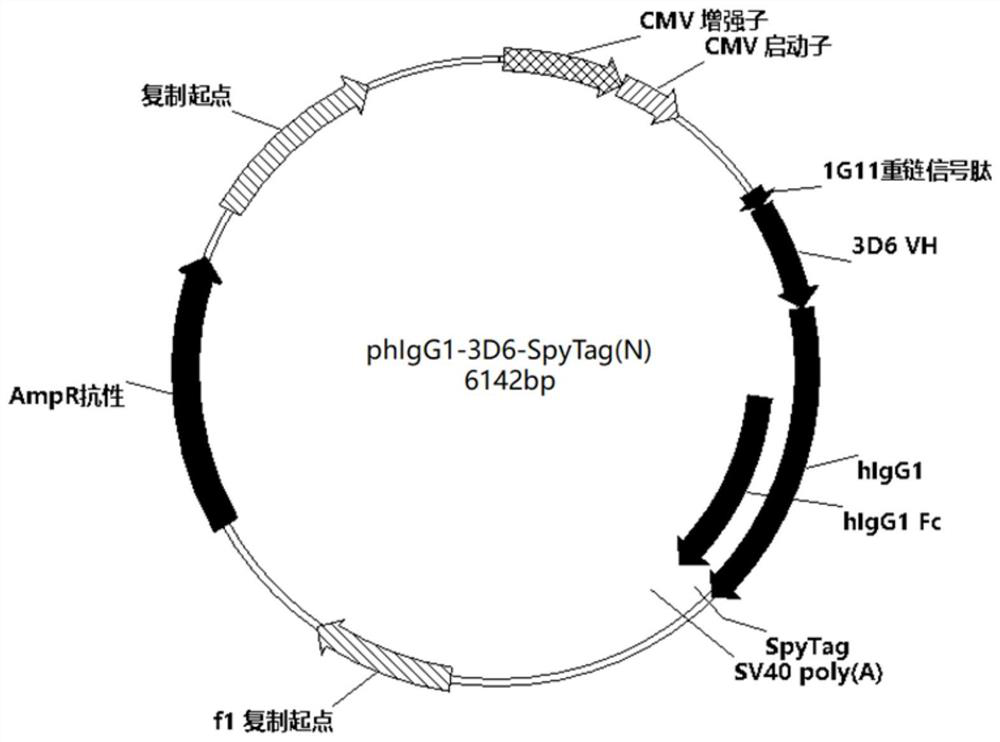
[0051] The mammalian codon-optimized sequence (SEQ ID NO: 7) of the amino acid sequence (SEQ ID NO: 6) of the signal peptide of the heavy chain of 1G11 was provided by Suzhou Industrial Park Weida Biotechnology Co., Ltd. Using phIgG1-3D6-SpyTag (self-constructed in Example 1) as a template, design primers (Table 3), amplify the 1G11SP-3D6-SpyTag gene fragment by PCR, then recover the corresponding fragment from the gel, and clone it into the phIgG1-3D6-SpyTag vector Between the multiple cloning sites on EcoR I and Hind III, a heavy chain expression vector phIgG1-3D6-SpyTag (N) capable of expressing the fusion protein 3D6-SpyTag (N) was constructed (plasmid map as shown in image 3 ), which were correctly identified by sequencing and entered into the library. The carrier phIgG1-3D6-SpyTag (N) was identified with restriction endonucleases EcoR I and Hind III (enzyme digestion...
Embodiment 3
[0056] Example 3 Construction of antibody fusion protein 3D6-SpyTag(N) light chain expression vector
[0057] 1G11 light chain signal peptide amino acid sequence (SEQ ID NO: 11) mammalian codon optimized sequence (SEQ ID NO: 12) and 3D6 (anti-human DEC205 monoclonal antibody) light chain expression vector phIgK-3D6 (signal peptide is mouse IgG1 signal peptide, amino acid sequence (SEQ ID NO: 3) was provided by Suzhou Industrial Park Weida Biotechnology Co., Ltd. Using phIgK-3D6 as a template, design primers (Table 5), amplify the 1G11SP-3D6VL-hIgkappa gene fragment by PCR, then recover the corresponding fragment from the gel, and clone it into the multiple cloning site EcoR I and HindIII on the phIgK-3D6 vector. In between, the light chain expression vector phIgK-3D6(N2) that can express the fusion protein 3D6-SpyTag(N) was constructed (plasmid map as Figure 5 ), which were correctly identified by sequencing and entered into the library. The carrier phIgK-3D6(N2) was identi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com