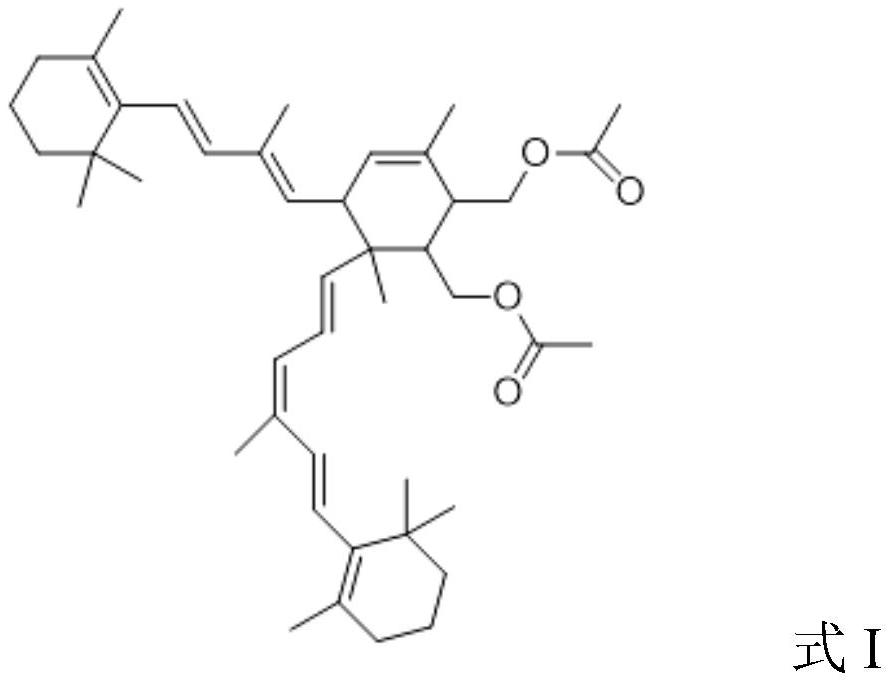
Vitamin A isomerization method
A vitamin and isomerization technology, applied in the field of isomerization, can solve the problems of increased 9-cis vitamin A content, inability to isomerize vitamin A, and difficulty in isomerization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Follow the steps below to carry out the isomerization reaction:
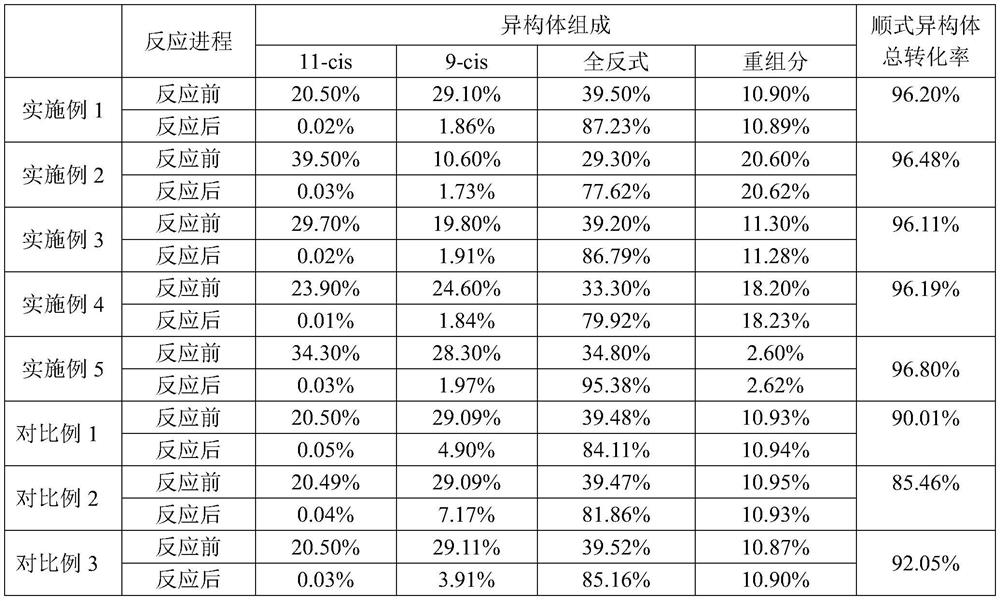
[0057] The dimer content in the VA crude oil A was adjusted to 0.0011%; 100 g of the adjusted VA crude oil was weighed, and 1900 g of dichloromethane was added to prepare a mixed solution. Add 0.0010 g of ruthenium trichloride, and replace the reaction system with nitrogen for 10 min, then stir and react at 20° C. for 10 h. After the reaction, the composition of the reaction solution was analyzed by high performance liquid chromatography, and the results of the isomerization reaction are listed in Table 1.
Embodiment 2
[0059] Follow the steps below to carry out the isomerization reaction:
[0060] The dimer content in VA crude oil B was adjusted to 0.0015%; 110 g of the adjusted VA crude oil was weighed, and 165 g of n-hexane was added to prepare a mixed solution. Add 0.0043g of rhodium acetate, replace the reaction system with nitrogen for 10 minutes, and then stir and react at 40°C for 4 hours. After the reaction, analyze the composition of the reaction solution by high performance liquid chromatography.
Embodiment 3
[0062] Follow the steps below to carry out the isomerization reaction:
[0063] Adjust the dimer content in VA crude oil C to 0.0112%; weigh 90g of the adjusted VA crude oil, add 210g of acetonitrile to prepare a mixed solution; then add 0.4099g of potassium tetrachloropalladate, and replace the reaction system with nitrogen for 10 minutes , stirred and reacted at 50° C. for 3 h. After the reaction, the composition of the reaction solution was analyzed by high performance liquid chromatography. The results of the isomerization reaction are listed in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com