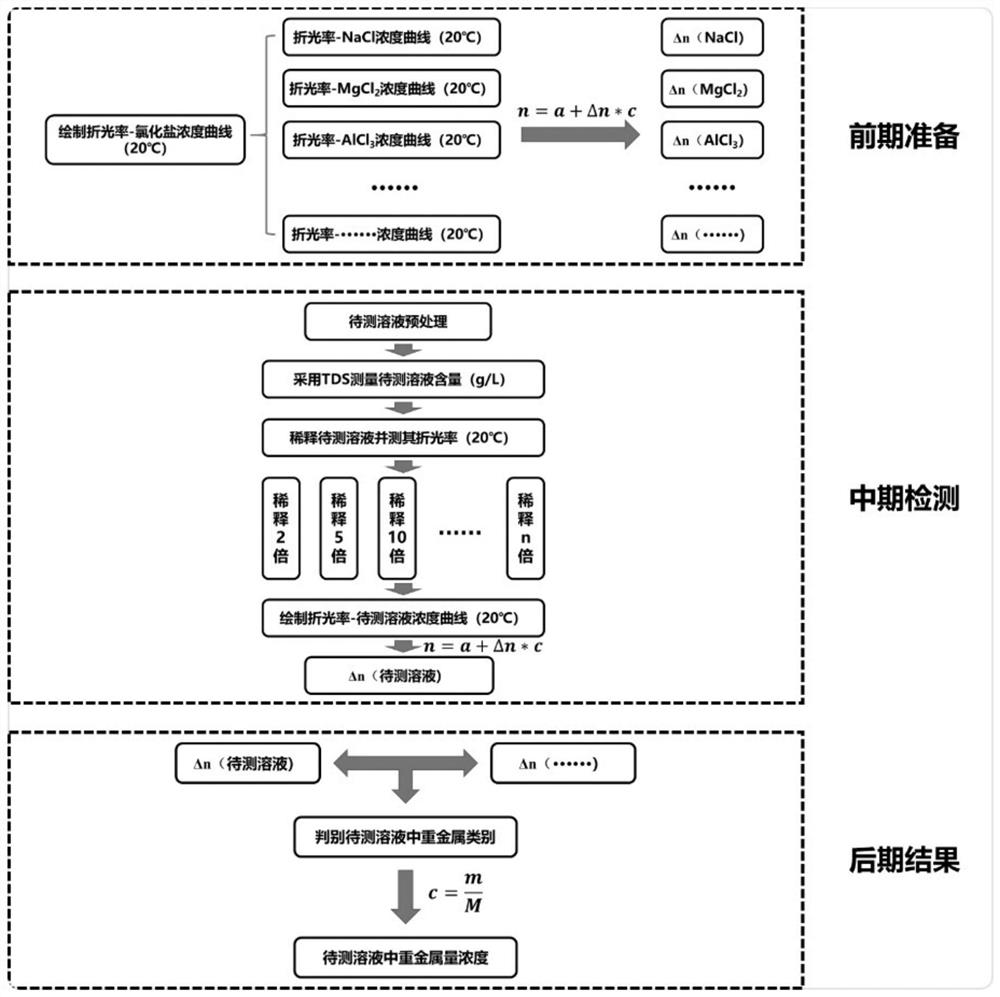
Method for detecting metal ions in aqueous solution based on refractive index change rate
A technology of refractive index and aqueous solution, which is applied in the field of heavy metal detection, can solve problems such as detection of metal ions that have not yet been seen, and achieve the effect of simple operation, short time consumption and less acquisition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
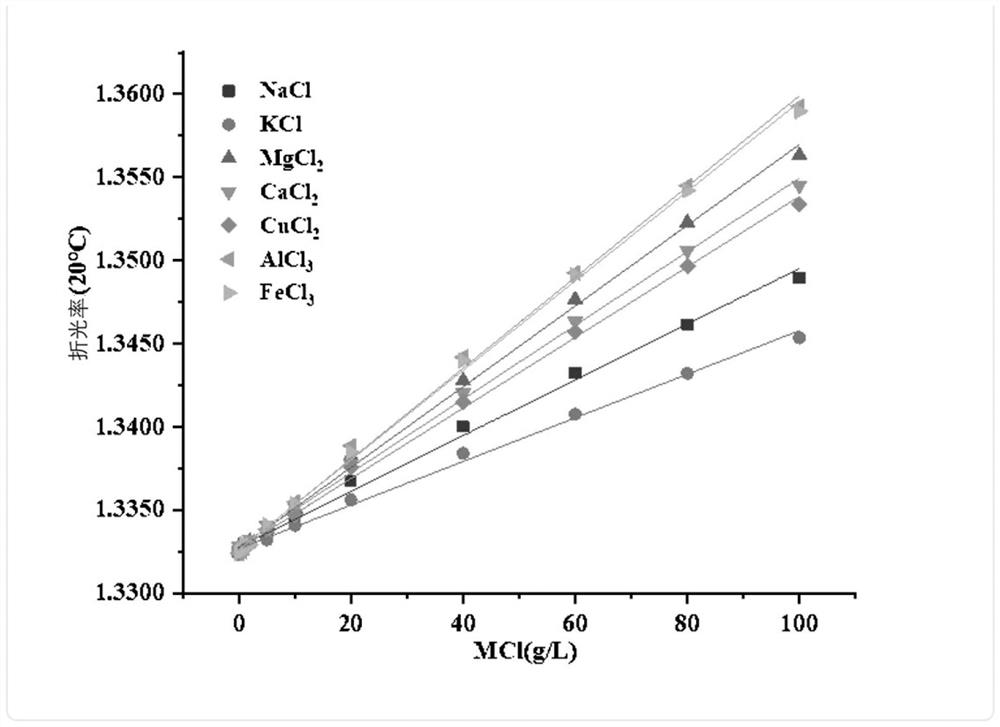
[0045]1. Draw the refractive index-sodium chloride concentration curve at 20°C: configure the solvent as pure water in sequence, and the concentration is 0, 0.1g / L, 0.2g / L, 0.5g / L, 1.0g / L, 2.0g / L L, 5.0g / L, 10.0g / L, 20.0g / L, 40.0g / L, 60.0g / L, 80.0g / L, 100.0g / L sodium chloride aqueous solution, measured by Abbe refractometer Refractive index at 20°C, draw the curve and fit it with n=a+Δn*c model to get Δn(Na + );
[0046] Repeat the above operations, draw the curves of refractive index-potassium chloride, refractive index-magnesium chloride, refractive index-calcium chloride, refractive index-copper chloride, refractive index-aluminum chloride, and refractive index-ferric chloride successively, with n =a+Δn*c model fitting to get Δn(K + ), Δn(Mg 2+ ), Δn(Ca 2+ ), Δn(Cu 2+ ), Δn(Al 3+ ), Δn(Fe 3+ ), the summary results are shown in Table 1;
[0047] the solution linear fit R 2
Δn NaCl y=1.33279+0.000167455x 0.99612 0.000167455±3.01488·10 -6
...
Embodiment 2
[0055] 1. The steps are the same as Example 1
[0056] 2. Pretreat the sample to be tested (a chlorinated saline solution), and use TDS to measure its solid content concentration (the original concentration of the sample to be tested is 50g / L);
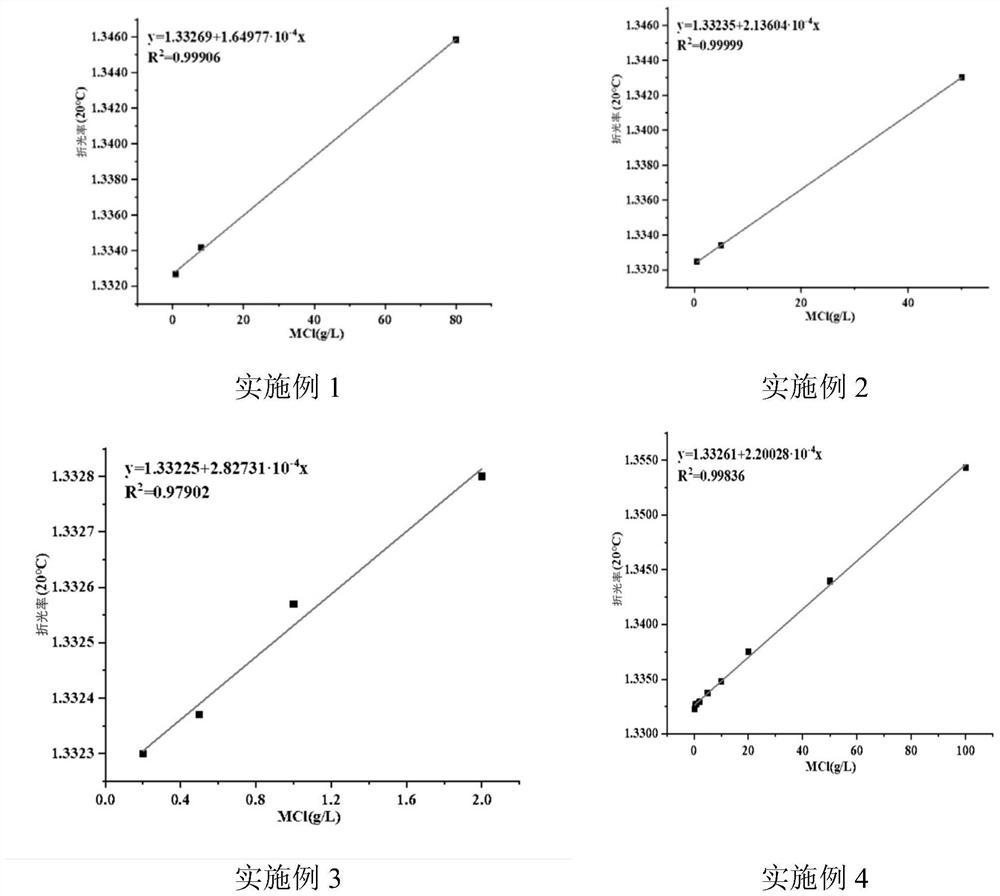
[0057] 3. Draw the refractive index-concentration curve of the sample to be tested at 20°C: dilute the sample to be tested 10 times and 100 times in turn, and dilute it 10 times (5g / L) as it is (50g / L), and dilute it 100 times (0.5 g / L) as the experimental group, the Abbe refractometer was used to measure its refractive index at 20°C, the refractive index was 1.34303, 1.33340, 1.33247 in turn, and the curve was drawn and fitted with n=a+Δn*c model to obtain Δn =2.13604·10 -4 ;Such as image 3 shown.
[0058] 4. Match the Δn of the sample to be tested with the Δn of various known solutions in Table 1, and it can be determined that the sample to be tested is a copper chloride solution;
[0059] 5. Step 3 measures the content of the ...
Embodiment 3
[0061] 1. The steps are the same as Example 1
[0062] 2. Pretreat the sample to be tested (a chlorinated saline solution), and use TDS to measure its concentration (the original concentration of the sample to be tested is 2g / L);
[0063] 3. Draw the refractive index-concentration curve of the sample to be tested at 20°C: Dilute the sample to be tested by 2 times, 4 times, and 10 times in sequence, and dilute it as it is (2g / L), dilute it by 2 times (1g / L), and dilute it by 4 times. times (0.5g / L), diluted 10 times (0.2g / L) as the experimental group, using the Abbe refractometer to measure its refractive index at 20 ℃, the refractive index is 1.3328, 1.33257, 1.33237, 1.3323, and draw The curve is fitted with n=a+Δn*c model to get Δn=2.82731·10 -4 ±2.38136·10 -5 ;Such as image 3 shown.
[0064] 4. Match the Δn of the sample to be tested with the Δn of various known solutions in Table 1, and it can be determined that the sample to be tested is an aluminum chloride solution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap