Chimeric receptor and application thereof
A chimeric receptor and receptor technology, applied in the field of biomedicine, can solve the problems of weakening antibody-dependent cell-mediated cytotoxicity and low affinity, and achieve the effect of enhancing ADCC effect, high affinity, and avoiding functional impairment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
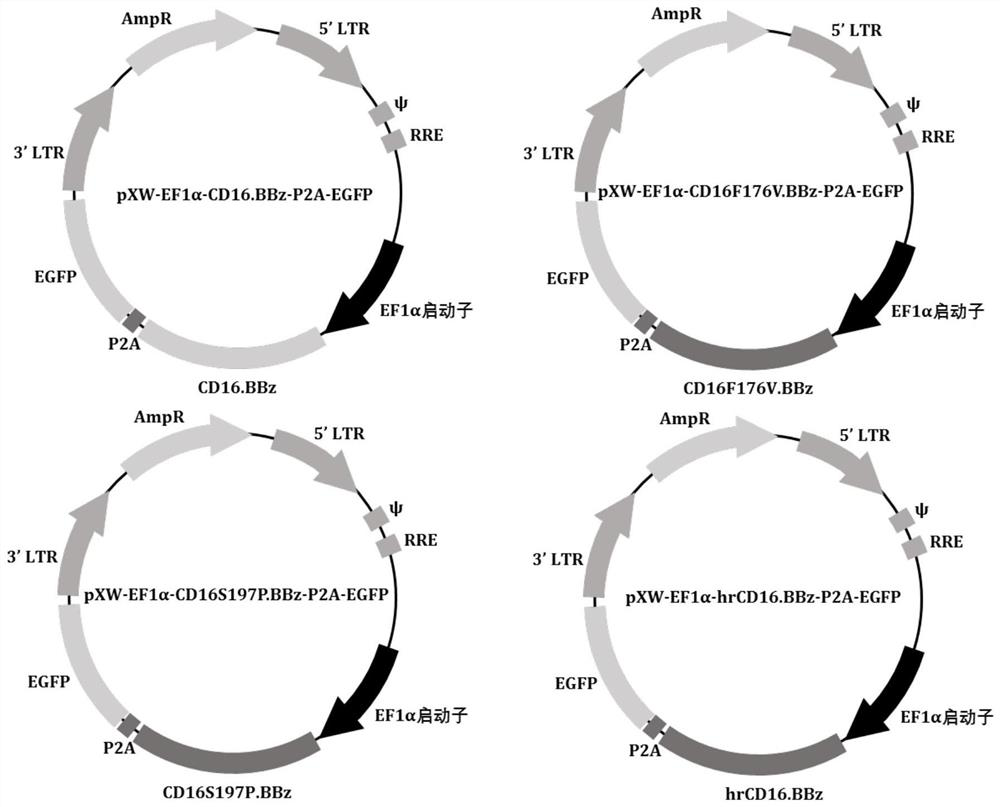
[0114] The wild-type CD16.BBz, F176V mutant CD16.BBz, S197P mutant CD16.BBz and hrCD16.BBz genes (respectively shown in SEQ ID NO: 13-16) were synthesized by Shanghai Jierui Bioengineering Co., Ltd., and cloned into blank slow Virus expression plasmids (pXW-EF1α-MCS-P2A-EGFP) were obtained pXW-EF1α-CD16.BBz-P2A-EGFP, pXW-EF1α-CD16F176V.BBz-P2A-EGFP, pXW-EF1α-CD16S197P.BBz-P2A- EGFP and pXW-EF1α-hrCD16.BBz-P2A-EGFP recombinant lentiviral expression plasmid, the plasmid map is as follows figure 1 shown.
Embodiment 2
[0115] Example 2 Lentiviral expression plasmid transfection of HEK 293T cells
[0116] The day before the experiment, seed HEK293T cells on a 12-well flat-bottomed cell culture plate, 4×10 5cells / 2mL / well. On the next day, the pXW-CD16.BBz and XW-hrCD16.BBz lentiviral expression plasmids were transfected with TurboFect transfection reagent, and the total amount of plasmids was 2 μg / well. Add 4 μL of TurboFect transfection reagent at a ratio of plasmid amount (μg): transfection reagent (μL) = 1:2, and add the freshly prepared plasmid transfection complex to the above cell culture plate after incubating at room temperature for 15-20 min. Place at 37°C, 5% CO 2 Continue to culture under the conditions for 48 hours, centrifuge at 500×g at room temperature for 5 minutes, discard the supernatant, and collect the cells for later use.
Embodiment 3
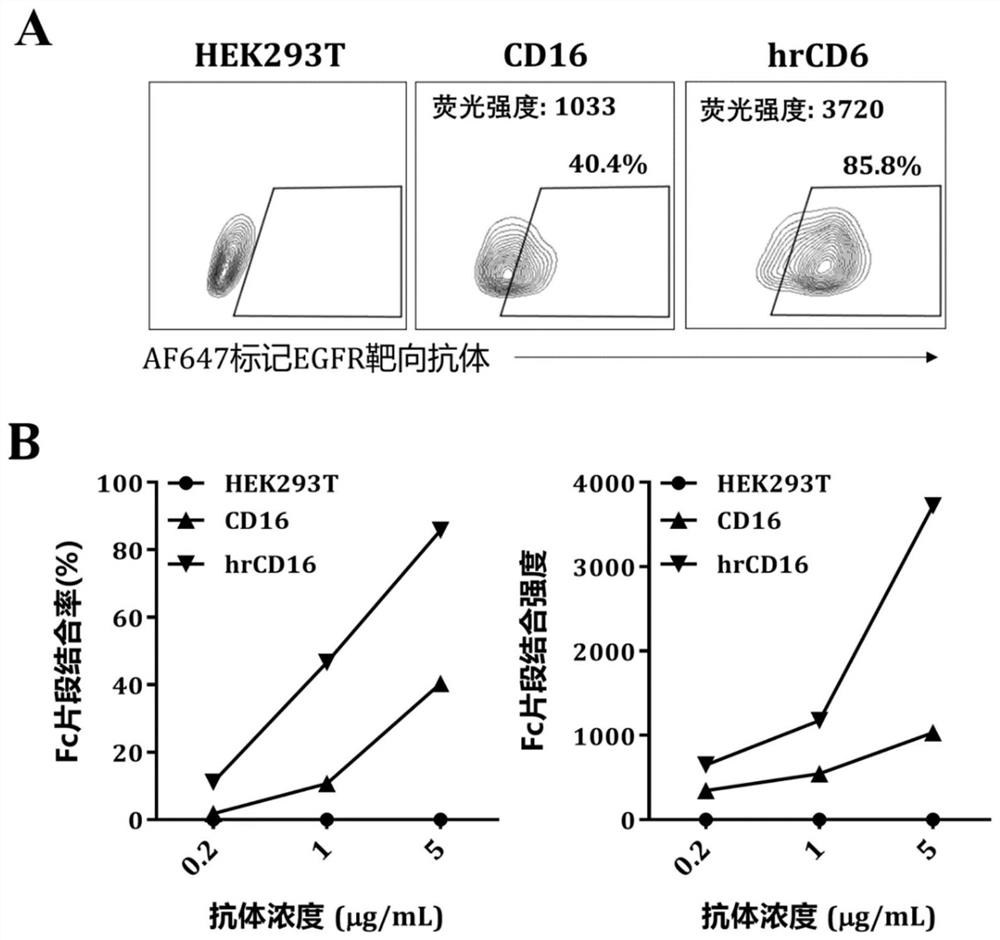
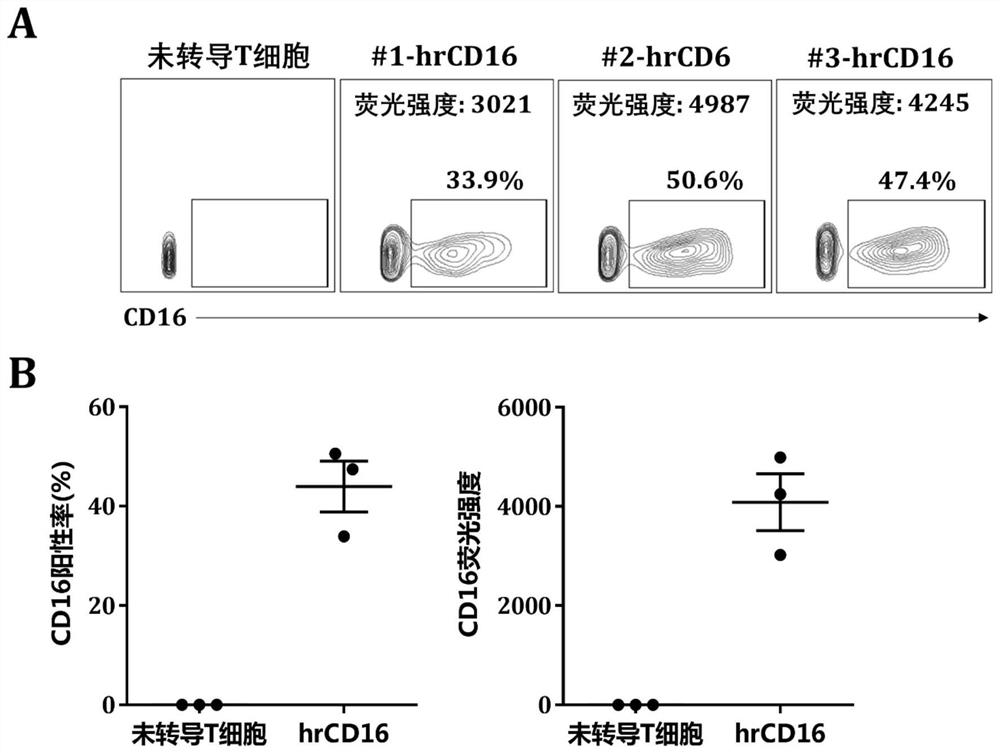
[0117] Example 3 hrCD16 chimeric receptor efficiently binds antibody Fc fragment
[0118] HEK293T cells expressing wild-type CD16 chimeric receptor (CD16.BBz) and hrCD16 chimeric receptor (hrCD16.BBz) were respectively prepared by the method described in Example 2.
[0119] Take 1×10 respectively 5 A wild-type CD16 chimeric receptor and hrCD16 chimeric receptor-modified HEK293T cells were placed in a 1.5mL EP tube, and EGFR-targeting antibody labeled with fluorescent dye AF647 was added, and the working concentration of the antibody was adjusted to 0.5, 1, and 5 μg / mL , incubated at 4° C. in the dark for 30 min, and then eluted twice with FACS buffer (1×PBS containing 2% FBS), resuspended in 200-300 μL of FACS buffer and detected by flow cytometry.
[0120] The result is as follows: figure 2 A is the flow diagram of HEK293T cells expressing wild-type CD16 chimeric receptor and hrCD16 chimeric receptor bound to the Fc fragment of EGFR-targeting antibody respectively. At t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com